Summary:
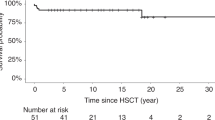
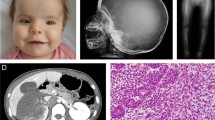
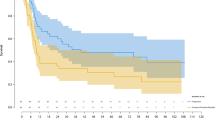
A retrospective analysis was made of 122 children who had received an allogeneic haematopoietic stem cell transplantation (HSCT) for autosomal recessive osteopetrosis between 1980 and 2001. The actuarial probabilities of 5 years disease free survival were 73% for recipients of a genotype HLA-identical HSCT (n=40), 43% for recipients of a phenotype HLA-identical or one HLA-antigen mismatch graft from a related donor (n=21), 40% for recipients of a graft from a matched unrelated donor (n=20) and 24% for patients who received a graft from an HLA-haplotype-mismatch related donor (n=41). In the latter group, a trend towards improvement was achieved at the end of the study period (17% before 1994, 45% after 1994, P=0.11). Causes of death after HSCT were graft failure and early transplant-related complications. Severe visual impairment was present in 42% of the children before HSCT. Conservation of vision was better in children transplanted before the age of 3 months. Final height was related to height at the time of HSCT and better preserved in children transplanted early. Most children attended regular school or education for the visually handicapped. At present, HSCT is the only curative treatment for autosomal recessive osteopetrosis and should be offered as early as possible.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Gerritsen EJA, Vossen JM, Loo IHG van et al. Autosomal recessive osteopetrosis: variability of findings at diagnosis and during the natural course. Pediatrics 1994; 93: 247–253.
Frattini A, Orchard PJ, Sobacchi C et al. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis (Letter). Nat Genet 2000; 25: 343–346.
Sobacchi C, Frattini A, Orchard P et al. The mutational spectrum of human malignant autosomal recessive osteopetrosis. Hum Mol Genet 2001; 10: 1767–1773.
Sly WS . The carbonic anhydrase II deficiency syndrome: osteopetrosis with renal tubular acidosis and cerebral calcifications. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds). The Metabolic Basis of Inherited Disease, vol. II, McGraw-Hill: New York, 2001, pp 5331–5343.
Kornak U, Kasper D, Bosl MR et al. Loss of the CIC-7 chloride channel leads to osteopetrosis in mice and man. Cell 2001; 104: 205–215.
Ballet JJ, Griscelli C, Coutris C et al. Bone-marrow transplantation in osteopetrosis. Lancet 1977; 2: 1137 (Letter).
Coccia PF, Krivit W, Cervenka J et al. Successful bone-marrow transplantation for infantile malignant osteopetrosis. N Engl J Med 1980; 302: 701–708.
Sorell M, Kapoor N, Kirkpatrick D et al. Marrow transplantation for juvenile osteopetrosis. Am J Med 1981; 70: 1280–1287.
Seiff CA, Chessels JM, Levinsky RJ et al. Allogeneic bone-marrow transplantation in infantile malignant osteopetrosis. Lancet 1983; 1: 437–441.
Gerritsen EJ, Vossen JM, Fasth A et al. Bone marrow transplantation for autosomal recessive osteopetrosis: a report from the working party on inborn errors of the European bone marrow transplantation group. J Pediatr 1994; 125: 896–902.
Key L, Carnes D, Cole S et al. Treatment of congenital osteopetrosis with high-dose calcitriol. N Engl J Med 1984; 310: 409–415.
Key LL, Rodriquiz RM, Willi SM et al. Long-term treatment of osteopetrosis with recombinant human interferon gamma. N Engl J Med 1995; 332: 1594–1599.
Reeves JD, Huffer WE, August CS et al. The hematopoietic effects of prednisone therapy in four infants with osteopetrosis. J Pediatr 1979; 94: 210–214.
Dorantes LM, Mejia AM, Dorantes S . Juvenile osteopetrosis: effects on blood and bone of prednisone and a low calcium, high phosphate diet. Arch Dis Child 1986; 61: 666–670.
Fischer A, Friedrich W, Fasth A et al. Reduction of graft failure by a monoclonal antibody (anti-LFA-1 CD11a) after HLA nonidentical bone marrow transplantation in children with immunodeficiencies, osteopetrosis, and Fanconi's anemia: a European Group for Immunodeficiency/European Group for Bone Marrow Transplantation report. Blood 1991; 77: 249–256.
Jabado N, Le Deist F, Cant A et al. Bone marrow transplantation from genetically HLA-nonidentical donors in children with fatal inherited disorders excluding severe combined immunodeficiencies: use of two monoclonal antibodies to prevent graft rejection. Pediatrics 1996; 98: 420–428.
Cobbold S, Martin G, Waldmann H . Monoclonal antibodies for the prevention of graft-versus-host disease and marrow graft rejection. The depletion of T cell subsets in vitro and in vivo. Transplantation 1986; 42: 239–247.
Locatelli F, Beluffi G, Georgiani G et al. Transplantation of cord blood progenitor cells can promote bone resorption in autosomal recessive osteopetrosis. Bone Marrow Transplant 1997; 20: 701–705.
Schulz AS, Classen CF, Mihatsch WA et al. HLA-haploidentical blood progenitor cell transplantation in osteopetrosis. Blood 2002; 99: 3458–3460.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Freeman JV, Cole TJ, Chinn S et al. Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child 1995; 73: 17–24.
Thompson DA, Kriss A, Taylor D et al. Early VEP and ERG evidence of visual dysfunction in autosomal recessive osteopetrosis. Neuropediatrics 1998; 29: 137–144.
Haines SJ, Erickson DL, Wirtschafer JD . Optic nerve decompression for osteopetrosis in early childhood. Neurosurgery 1988; 23: 470–475.
Rees H, Ang LC, Casey R, George DH . Association of infantile neuroaxonal dystrophy and osteopetrosis: a rare autosomal recessive disorder. Pediatr Neurosurg 1995; 22: 321–327.
Abinun M, Newson T, Rowe PW et al. Importance of neurological assessment before bone marrow transplantation for osteopetrosis. Arch Dis Child 1999; 80: 273–274.
Kapelushnik J, Shalev C, Yaniv I et al. Osteopetrosis: a single center experience of stem cell transplantation and prenatal diagnosis. Bone Marrow Transplant 2001; 27:129–132.
Flanagan AM, Sarma U, Steward CG et al. Study of the nonresorptive phenotype of the osteoclast-like cells from patients with malignant osteopetrosis: a new approach to investigating pathogenesis. J Bone Miner Res 2000; 15: 352–360.
Helfrich MH, Gerritsen EJA . Formation of non-resorbing osteoclasts from peripheral blood mononuclear cells of patients with malignant juvenile osteopetrosis. Br J Hematol 2001; 112: 64–68.
Flanagan AM, Massey HM, Wilson C et al. Macrophage colony-stimulating factor and receptor activator NF-kappa B ligand fail to rescue osteoclast-poor human malignant infantile osteopetrosis in vitro. Bone 2002; 30: 85–90.
Marks SC . Osteopetrosis: multiple pathways for the interception of osteoclast function. Bone Pathology Appl Pathol 1987; 5: 172–183.
Teitelbaum SL . Bone resorption by osteoclasts. Science 2000; 289: 1504–1508.
Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci USA 1990; 87: 4828–4832.
Droz-Desprez D, Azou C, Bordigoni P, Bonnaure-Mallet M . Infantile osteopetrosis: a case report on dental findings. J Oral Pathol Med 1992; 21: 422–425.
Jalevik B, Fasth A, Dahlof G . Dental development after successful treatment of infantile osteopetrosis with bone marrow transplantation. Bone Marrow Transplant 2002; 29: 537–540.
Acknowledgements
The following centres contributed to this study: Hacettepe Children's Hospital, Ankara, Turkey (Dr D Uckan); Department of Pediatrics, University Hospital Brescia, Italy (Dr F Porta); Royal Hospital for Children, Bristol, U K (Dr C Steward); Hôspital Universitaire des Enfants, Brussels, Belgium (Dr A Ferster); Our Lady's Hospital for Sick Children, Dublin, Ireland (Dr A O’Meara); Department of Hematology, San Martino Hospital, Genova, Italy (Dr A Bacigalupo); Department of Pediatric Hemato-oncology, University Hospital Gent, Belgium (Dr Y Benoit); Royal Hospital for Sick Children, Glasgow, Scotland (Dr B Gibson); Queen Silvia Children's Hospital, Göteborg, Sweden (Dr A Fasth); Department of Pediatrics, University Hospital Leiden, The Netherlands (Dr J Vossen); Hospital for Sick Children, London, U K (Drs P Veys and A Vellodi); Hôpital d’Enfants, Nancy, France (Dr P Bordigoni); Department of Pediatrics, General Hospital, Newcastle, U K (Dr A Cant); Department of Immunology and Hematology, Hôpital Necker Enfants Malades, Paris, France (Drs A Fischer and C Griscelli); Hôspital St Louis, Paris, France (Dr E Gluckman); Hôpital Debré, Paris, France (Dr E Vilmer); Clinica Pediatrica, Pavia, Italy (Dr F Locatelli); Department of Oncology, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia (Drs P Ernst and A Padmos); Department of Medicine, National Children's Hospital, San Jóse, Costa Rica (Dr O Porras); University Children's Hospital, Ulm, Germany (Dr W Friedrich).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Driessen, G., Gerritsen, E., Fischer, A. et al. Long-term outcome of haematopoietic stem cell transplantation in autosomal recessive osteopetrosis: an EBMT report. Bone Marrow Transplant 32, 657–663 (2003). https://doi.org/10.1038/sj.bmt.1704194
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704194
Keywords
This article is cited by
-
Haploidentical haematopoietic stem cell transplantation for malignant infantile osteopetrosis and intermediate osteopetrosis: a retrospective analysis of a single centre
Orphanet Journal of Rare Diseases (2021)
-
Genotyping, generation and proteomic profiling of the first human autosomal dominant osteopetrosis type II-specific induced pluripotent stem cells
Stem Cell Research & Therapy (2019)
-
Guided growth for valgus deformity correction of knees in a girl with osteopetrosis: a case report
Strategies in Trauma and Limb Reconstruction (2017)
-
Case update on cranial osteopetrosis: which is the role of the neurosurgeon?
Child's Nervous System (2017)