Abstract
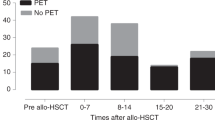
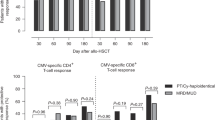
The aim of this study was to investigate the effects of HHV-6 DNAemia on the CMV specific lymphoproliferative response after allogeneic stem cell transplantation. Twenty-one allogeneic stem cell transplantation (allo-SCT) patients were included in the study. The patients were either CMV seropositive and/or had CMV seropositive donors. We studied the effects of HHV-6 infection, documented by PCR, on CMV-specific lymphocyte proliferation response and on CMV infection documented by PCR. HHV-6 DNAemia correlated with the absence of CMV-specific lymphocyte proliferation responses after allo-SCT. Three of nine patients with persistent HHV-6 DNA had a CMV-specific lymphocyte proliferative response compared to 11 of 12 patients without persistent HHV-6 DNAemia (P = 0.02). Patients with higher HHV-6 DNA levels (>100 copies) were more likely than those with lower copy numbers not to develop a CMV-specific immune response (six of nine vs one of eight; P < 0.05). Patients who were repeatedly HHV-6 positive in three or more consecutive blood samples were also more likely to need repeated courses of preemptive antiviral therapy against CMV during the first 6 months after transplantation (P < 0.001). Our data indicate the possibility that HHV-6 can suppress the development of CMV-specific immune responses and thereby could predispose to development of late CMV disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ljungman P, Aschan J, Lewensohn-Fuchs I et al. Results of different strategies for reducing cytomegalovirus-associated mortality in allogeneic stem cell transplant recipients Transplantation 1998 66: 1330 1334
Ljungman P . Cytomegalovirus infections in transplant patients Scand J Infect Dis 1996 100: (Suppl.) 59 63
Salzberger B, Bowden RA, Hackman RC et al. Neutropenia in allogeneic marrow transplant recipients receiving ganciclovir for prevention of cytomegalovirus disease: risk factors and outcome Blood 1997 90: 2502 2508
Li CR, Greenberg PD, Gilbert MJ et al. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis Blood 1994 83: 1971 1979
Wang FZ, Dahl H, Linde A et al. Lymphotropic herpesviruses in allogeneic bone marrow transplantation Blood 1996 88: 3615 3620
Ljungman P, Wang F-Z, Clark D et al. High levels of human herpesvirus 6 DNA in peripheral blood leukocytes are correlated to platelet engraftment and disease in allogeneic stem cell transplant patients Br J Haematol 2000 111: 774 781
Cone RW, Huang ML, Corey L et al. Human herpesvirus 6 infections after bone marrow transplantation: clinical and virologic manifestations J Infect Dis 1999 179: 311 318
Kadakia MP, Rybka WB, Stewart JA et al. Human herpesvirus 6: infection and disease following autologous and allogeneic bone marrow transplantation Blood 1996 87: 5341 5354
DesJardin JA, Gibbons L, Cho E et al. Human herpesvirus 6 reactivation is associated with cytomegalovirus infection and syndromes in kidney transplant recipients at risk for primary cytomegalovirus infection J Infect Dis 1998 178: 1783 1786
Dockrell DH, Prada J, Jones MF et al. Seroconversion to human herpesvirus 6 following liver transplantation is a marker of cytomegalovirus disease J Infect Dis 1997 176: 1135 1140
Reusser P, Riddell SR, Meyers JD, Greenberg PD . Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease Blood 1991 78: 1373 1380
Ljungman P, Aschan J, Azinge JN et al. Cytomegalovirus viraemia and specific T-helper cell responses as predictors of disease after allogeneic marrow transplantation Br J Haematol 1993 83: 118 124
Horvat RT, Parmely MJ, Chandran B . Human herpesvirus 6 inhibits the proliferative responses of human peripheral blood mononuclear cells J Infect Dis 1993 167: 1274 1280
Flamand L, Gosselin J, Stefanescu I et al. Immunosuppressive effect of human herpesvirus 6 on T-cell functions: suppression of interleukin-2 synthesis and cell proliferation [published erratum appears in Blood 1995 Jul 1; 86: 418] Blood 1995 85: 1263 1271
Yasukawa M, Inoue Y, Ohminami H et al. Apoptosis of CD4+ T lymphocytes in human herpesvirus-6 infection J Gen Virol 1998 79: 143 147
Ringdén O, Ruutu T, Remberger M et al. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: a report from the Nordic Bone Marrow Transplantation Group Blood 1994 83: 2723 2730
Ringdén O, Remberger M, Persson U et al. Similar incidence of graft-versus-host disease using HLA-A, -B and -DR identical unrelated bone marrow donors as with HLA-identical siblings Bone Marrow Transplant 1995 15: 619 625
Ljungman P, Öberg G, Aschan J et al. Foscarnet for pre-emptive therapy of CMV infection detected by a leukocyte-based nested PCR in allogeneic bone marrow transplant patients Bone Marrow Transplant 1996 18: 565 568
Wang FZ, Linde A, Dahl H, Ljungman P . Human herpesvirus 6 infection inhibits specific lymphocyte proliferation responses and is related to lymphocytopenia after allogeneic stem cell transplantation Bone Marrow Transplant 1999 24: 1201 1206
Linde A, Fridell E, Dahl H et al. Effect of primary Epstein–Barr virus infection on human herpesvirus 6, cytomegalovirus, and measles virus immunoglobulin G titers J Clin Microbiol 1990 28: 211 215
Ehrnst A, Barkholt L, Levensohhn-Fuchs I et al. CMV PCR monitoring in leukocytes of transplant patients Clin Diag Virol 1995 3: 139 153
Clark D, Ait-Khaled M, Wheeler A et al. Quantification of human herpesvirus 6 in immunocompetent persons and post-mortem tissues from AIDS patients by PCR J. Gen Virol 1996 77: 2271 2275
Thomas E, Bryant J, Buckner C et al. Allogenic marrow grafting using HL-A matched donor-recipient sibling pairs Trans Assoc Am Phys 1971 84: 248 261
Meyers J, Ljungland P, Fisher L . Cytomegalovirus excretion as a predictor of cytomegalovirus disease after marrow transplantation: importance of cytomegalovirus viremia J Infect Dis 1990 162: 373 380
Meyers J, Flournoy N, Thomas E . Risk factors for cytomegalovirus infection infection after human marrow transplantation J Infect Dis 1986 153: 478 488
Miller W, Flynn P, McCullough J et al. Cytomegalovirus infection after bone marrow transplantation: an association with acute graft-v-host disease Blood 1986 67: 1162 1167
Takenaka K, Gondo H, Tanimoto K et al. Increased incidence of cytomegalovirus (CMV) infection and CMV-associated disease after allogeneic bone marrow transplantation from unrelated donors. The Fukuoka Bone Marrow Transplantation Group Bone Marrow Transplant 1997 19: 241 248
Krause H, Hebart H, Jahn G et al. Screening for CMV-specific T cell proliferation to identify patients at risk of developing late onset CMV disease Bone Marrow Transplant 1997 19: 1111 1116
Borysiewicz L, Morris S, Page J, Sissons J . Human cytomegalovirus-specific cytotoxic T lymphocytes: requirements for in vitro generation and specificity Eur J Immunol 1983 13: 804 809
Walter EA, Greenberg PD, Gilbert MJ et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor New Engl J Med 1995 333: 1038 1044
Ljungman P, Lonnqvist B, Wahren B et al. Lymphocyte responses after cytomegalovirus infection in bone marrow transplant recipients – a one-year follow-up Transplantation 1985 40: 515 520
Meyers J, Flournoy N, Thomas E . Cytomegalovirus infection and specific cell-mediated immunity after marrow transplant J Infect Dis 1980 142: 816 824
Einsele H, Ehninger G, Steidle M et al. Polymerase chain reaction to evaluate antiviral therapy for cytomegalovirus disease Lancet 1991 ii: 1170 1172
Söderberg C, Larsson S, Bergstedt-Lindqvist S, Möller E . Definition of a subset of human peripheral blood mononuclear cells that are permissive to human cytomegalovirus infection J Virol 1993 67: 3166 3175
Acknowledgements
This study was supported by the Swedish Children's Cancer Fund and the Swedish Cancer Society. We are grateful to Vince Emery and Duncan A Clark at Royal Free Hospital School of Medicine for help with the quantitative, competitive PCR analyses. We also thank Kirsti Niemele, RN, and Mari Svensson, RN, at Huddinge University Hospital for collecting the samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, FZ., Larsson, K., Linde, A. et al. Human herpesvirus 6 infection and cytomegalovirus-specific lymphoproliferative responses in allogeneic stem cell transplant recipients. Bone Marrow Transplant 30, 521–526 (2002). https://doi.org/10.1038/sj.bmt.1703657
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703657