Abstract
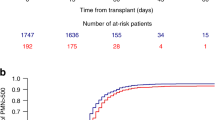
Factors influencing hematopoietic recovery (HR) after autologous blood stem cell transplantation (ABSCT) were analyzed in 73 patients with various non-myeloid malignancies (NMM), and in 58 patients with acute myeloblastic leukemia (AML). Peripheral blood stem cells were collected following mobilization with chemotherapy, granulocyte colony-stimulating factor (G-CSF), or chemotherapy plus G-CSF. The conditioning regimen used consisted of either chemotherapy alone (112 cases) or chemotherapy plus total body irradiation (19 cases). The median number of colony-forming units granulocyte–macrophage (CFU-GM) was similar in both groups of patients, with the median number of CD34+ cells infused being higher in the AML group (5.4 vs 4 × 106/kg; P = 0.03). Median time neutrophils >0.5 × 109/l was 13 days in both groups, and median time to a platelet count >20 × 109/l was longer in AML patients (14 vs 12 days; P = 0.01). In multivariate analysis, the only factors affecting neutrophil recovery in the NMM group were the CD34+ cell number (continuous model) and the CFU-GM dose (categorized model) infused, whereas for platelet recovery, previous chemotherapy also remained significant. In the AML group, the only factors significantly affecting the speed of neutrophil recovery were dose of CD34+ cells administered and the patient's age. As for platelet recovery, only the progenitor dose administered remained significant. In the NMM group, the most discriminating cut-off values for a rapid neutrophil and platelet recovery were 1.5 × 106 and 2.5 × 106 CD34+ cells/kg, respectively, and for AML patients these figures were 1.5 × 106 and 4 × 106 CD34+ cells/kg, respectively. Our results confirm the slower HR after ABSCT in AML, and highlight the importance of progenitor cell dose in accelerating HR after ABSCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bensinger W, Appelbaum F, Rowley S et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells J Clin Oncol 1995 13: 2547 2555
Weaver CH, Hazelton B, Birch R et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy Blood 1995 86: 3961 3969
Haas R, Witt B, Möhle R et al. Sustained long-term hematopoiesis after myeloablative therapy with peripheral blood progenitor cell support Blood 1995 85: 3754 3761
Pavlovsky S, Koziner B, Milone G et al. Multivariate analyses of prognostic factors associated with hematopoietic recovery in autograft patients with different sources of progenitor cells Ann Oncol 1996 7: 719 724
Lowenthal RM, Fabères C, Marit G et al. Factors influencing haemopoietic recovery following chemotherapy-mobilised autologous peripheral blood progenitor cell transplantation for haematological malignancies: a retrospective analysis of a 10-year single institution experience Bone Marrow Transplant 1998 22: 763 770
Sanz MA, de la Rubia J, Sanz GF et al. Busulfan plus cyclophosphamide followed by autologous blood stem-cell transplantation for patients with acute myeloblastic leukemia in first complete remission: a report from a single institution J Clin Oncol 1993 11: 1661 1667
de la Rubia J, Sanz GF, Martin G et al. Autologous blood stem cell transplantation for acute myeloblastic leukemia in first complete remission. Intensification therapy before transplantation does not prolong disease-free survival Haematologica 1999 84: 125 132
de la Rubia J, Sanz MA . Técnicas para la movilización y recolección de células hematopoyéticas circulantes Sangre 1991 36: 181 184
Iscove NN, Senn JS, Till JE, McCulloch EA . Colony formation by normal and leukemic human marrow cells in culture: effect of conditioned medium from human leukocytes Blood 1971 37: 1 5
Sutherland DR, Anderson L, Keeney M et al. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering J Hematother 1996 5: 213 226
de la Rubia J, Sanz GF, Martin G et al. Autologous bone marrow transplantation for patients with acute myeloblastic leukemia in relapse after autologous blood stem cell transplantation Bone Marrow Transplant 1996 18: 1167 1173
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations J Am Stat Assoc 1958 53: 457 481
Cox DR, Oakes DO . Analysis of Survival Data Chapman and Hall: London 1984
Adams JA, Gordon AA, Jiang YZ et al. Thrombocytopenia after bone marrow transplantation for leukaemia: changes in megakaryocyte growth and growth-promoting activity Br J Haematol 1990 75: 195 201
Chang J, Geary CG, Testa NG . Long-term bone marrow damage after chemotherapy for acute myeloid leukaemia does not improve with time Br J Haematol 1990 75: 68 72
Straetmans N, Ma DD, Herman P et al. Long-term culture of autologous transplanted bone marrow for acute myeloid leukaemia: evidence for an in vitro haemopoietic defect and lack of correlation with the speed of engraftment Bone Marrow Transplant 1995 15: 421 428
Carlo-Stella C, Tabilio A, Regazzi E et al. Effect of chemotherapy for acute myelogenous leukemia on hematopoietic and fibroblast marrow progenitors Bone Marrow Transplant 1997 20: 465 471
Schwartzberg L, Birch R, Blanco R et al. Rapid and sustained hematopoietic reconstitution by peripheral blood stem cell infusion alone following high-dose chemotherapy Bone Marrow Transplant 1993 11: 369 374
Schwella N, Siegert W, Beyer J et al. Autografting with blood progenitor cells: predictive value of preapheresis blood cell counts on progenitor cell harvest and correlation of the reinfused cell dose with hematopoietic reconstitution Ann Hematol 1995 71: 227 234
Watts MJ, Sullivan AM, Jamieson E et al. Progenitor-cell mobilization after low-dose cyclophosphamide and granulocyte colony-stimulating factor: an analysis of progenitor-cell quantity and quality and factors predictive for these parameters in 101 pretreated patients with malignant lymphoma J Clin Oncol 1997 15: 535 546
Kiss JE, Rybka WB, Winkelstein A et al. Relationship of CD34+ cell dose to early and late hematopoiesis following autologous peripheral blood stem cell transplantation Bone Marrow Transplant 1997 19: 303 310
Marit G, Thiessard F, Faberes C et al. Factors affecting both peripheral blood progenitor cell mobilization and hematopoietic recovery following autologous blood progenitor cell transplantation in multiple myeloma patients: a monocentric study Leukemia 1998 12: 1447 1456
To LB, Haylock DN, Dyson PG et al. An unusual pattern of hemopoietic reconstitution in patients with acute myeloid leukemia transplanted with autologous recovery phase peripheral blood Bone Marrow Transplant 1990 6: 109 114
Demirer T, Buckner CD, Appelbaum FR et al. Rapid engraftment after autologous transplantation utilizing marrow and recombinant granulocyte colony-stimulating factor-mobilized peripheral blood stem cells in patients with acute myelogenous leukemia Bone Marrow Transplant 1995 15: 915 922
Gondo H, Harada M, Miyamoto T et al. Autologous peripheral blood stem cell transplantation for acute myelogenous leukemia Bone Marrow Transplant 1997 20: 821 826
Martin G, Torres A, León A et al. Autologous peripheral blood stem cell transplantation (PBSCT) mobilized with G-CSF in AML in first complete remission. Role of intensification therapy in outcome Bone Marrow Transplant 1998 21: 375 382
Vellenga E, Van Putten WL, Boogaerts MA et al. Peripheral blood stem cell transplantation as an alternative to autologous marrow transplantation in the treatment of acute myeloid leukemia Bone Marrow Transplant 1999 23: 1279 1282
Juttner CA, To LB, Haylock DN et al. The threshold effect in peripheral blood stem cell autografting – differences between acute myeloid leukemia and non-stem cell disease Exp Hematol 1989 17: 357a (Abstr.)
Ketterer N, Salles G, Raba M et al. High CD34(+) cell counts decrease hematologic toxicity of autologous peripheral blood progenitor cell transplantation Blood 1998 91: 3148 3155
Brugger W, Bross KJ, Glatt M et al. Mobilization of tumor cells and hematopoietic progenitor cells into peripheral blood of patients with solid tumors Blood 1994 83: 636 640
Altman DG, Lausen B, Sauerbrei W, Schumacher M . Dangers of using optimal cutpoints in the evaluation of prognostic factors J Natl Cancer Inst 1994 86: 829 835
Dreger P, Kloss M, Petersen B et al. Autologous progenitor cell transplantation: prior exposure to stem cell-toxic drugs determines yield and engraftment of peripheral blood progenitor cell but not of bone marrow grafts Blood 1995 86: 3970 3978
Tricot G, Jagannath S, Vesole D et al. Peripheral blood stem cell transplants for multiple myeloma: identification of favorable variables for rapid engraftment in 225 patients Blood 1995 85: 588 596
Bentley SA, Brecher ME, Powell E et al. Long-term engraftment failure after marrow ablation and autologous hematopoietic reconstitution: differences between peripheral blood stem cell and bone marrow recipients Bone Marrow Transplant 1997 19: 557 563
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carral, A., de la Rubia, J., Martín, G. et al. Factors influencing hematopoietic recovery after autologous blood stem cell transplantation in patients with acute myeloblastic leukemia and with non-myeloid malignancies. Bone Marrow Transplant 29, 825–832 (2002). https://doi.org/10.1038/sj.bmt.1703566
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703566
Keywords
This article is cited by
-
Implications of hematopoietic stem cells heterogeneity for gene therapies
Gene Therapy (2021)
-
Factors that predict delayed platelet recovery after autologous stem cell transplantation for lymphoma or myeloma
Annals of Hematology (2020)
-
Predictive factors for hematopoietic engraftment after autologous peripheral blood stem cell transplantation for AL amyloidosis
Bone Marrow Transplantation (2005)
-
Impact of different strategies of second-line stem cell harvest on the outcome of autologous transplantation in poor peripheral blood stem cell mobilizers
Bone Marrow Transplantation (2005)
-
Graft clonogenicity and intensity of pre-treatment: factors affecting outcome of autologous peripheral hematopoietic cell transplantation in patients with acute myeloid leukemia in first remission
Bone Marrow Transplantation (2005)