Abstract
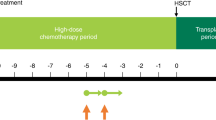
Topotecan appears to be relatively unaffected by the most common multidrug resistance mechanisms, may potentiate cytotoxicity of alkylators, has good penetration into the central nervous system, is active against a variety of neoplasms, and has myelosuppression as its paramount toxicity. We present our experience with a myeloablative regimen that includes topotecan. Twenty-one patients with poor-prognosis tumors and intact function of key organs received topotecan 2 mg/m2 by 30-min intravenous (i.v.) infusion on days −8, −7, −6, −5, −4; thiotepa 300 mg/m2 by 3 h i.v. infusion on days −8, −7, −6; and carboplatin by 4 h i.v. infusion on days −5, −4, −3 with a daily dose derived from the pediatric Calvert formula, using a targeted area under the curve of seven mg/ml* min (∼500 mg/m2/day). Stem cell rescue was on day 0. The patients were 1 to 29 (median 4) years old; 18 were in complete remission (CR) and three in partial remission (PR). Early toxicities were severe mucositis and erythema with superficial peeling in all patients and a seizure, hypertension, and renal insufficiency followed by veno-occlusive disease in one patient each. Post-transplant treatment included radiotherapy alone (four patients) or plus biological agents (11 patients with neuroblastoma). With a follow-up of 6+ to 32+ (median 11+) months, event-free survivors include 10/11 neuroblastoma patients (first CR), 4/5 brain tumor patients (second PR or CR), 1/3 patients with metastatic Ewing's sarcoma (first or second CR), and a patient transplanted for multiply recurrent immature ovarian teratoma; a patient with desmoplastic small round-cell tumor (second PR) had progressive disease at 8 months. Favorable results for disease control, manageable toxicity, and the antitumor profiles of topotecan, thiotepa, and carboplatin, support use of this three-drug regimen in the treatment of neuroblastoma and brain tumors; applicability to other tumors is still uncertain. Bone Marrow Transplantation (2001) 28, 551–556.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kushner BH, O'Reilly RJ, Mandell LR et al. Myeloablative combination chemotherapy without total body irradiation for neuroblastoma J Clin Oncol 1991 9: 274–279
Dunkel IJ, Boyett JM, Yates A et al. High-dose carboplatin, thiotepa, and etoposide with autologous stem-cell rescue for patients with recurrent medulloblastoma J Clin Oncol 1998 16: 222–228
Matthay KK, Villablanca JG, Seeger RC et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid New Engl J Med 1999 341: 1165–1173
Kushner BH, Meyers PA . How effective is dose-intensive/myeloablative therapy against Ewing's sarcoma/primitive neuroectodermal tumor metastatic to bone or bone marrow? The Memorial Sloan-Kettering Cancer Center experience and a literature review J Clin Oncol 2001 19: 870–880
Houghton PJ, Cheshire PJ, Myers L et al. Evaluation of 9-dimethylaminomethyl-10-hydroxycamptothecin against xenografts derived from adult and childhood solid tumors Cancer Chemother Pharmacol 1992 31: 229–239
Hendricks CB, Rowinsky EK, Grochow LB et al. Effect of P-glycoprotein expression on the accumulation and cytotoxicity of topotecan (SK&F 104864), a new camptothecin analogue Cancer Res 1992 52: 2268–2278
Kaufmann SH, Peereboom D, Buckwalter CA et al. Cytotoxic effects of topotecan combined with various anticancer agents in human cancer cell lines J Natl Cancer Inst 1996 88: 734–741
Baker SD, Heideman RL, Crom WR et al. Cerebrospinal fluid pharmacokinetics and penetration of continuous infusion topotecan in children with central nervous system tumors Cancer Chemother Pharmacol 1996 37: 195–202
Pratt CB, Stewart C, Santana VM et al. Phase 1 study of topotecan for pediatric patients with malignant solid tumors J Clin Oncol 1994 12: 539–543
Kretschmar C, Kletzel M, Murray K et al. Upfront phase II therapy with taxol and topotecan in untreated children with disseminated neuroblastoma: A Pediatric Oncology Group study Med Pediatr Oncol 1995 25: 243 (Abstr.)
Tubergen DG, Stewart CF, Pratt CB et al. Phase I trial and pharmacokinetic (PK) and pharmacodynamics (PD) study of topotecan using a five-day course in children with refractory solid tumors: a Pediatric Oncology Group study J Pediatr Hematol/Oncol 1996 18: 352–361
Vietti T, Crist W, Ruby E et al. Topotecan window in patients with rhabdomyosarcoma: an IRSG study Proc Am Soc Clin Oncol 1997 16: 510a (Abstr.)
Nitschke R, Parkhurst J, Sullivan J et al. Topotecan in pediatric patients with recurrent and progressive solid tumors: a Pediatric Oncology Group phase II study J Pediatr Hematol/Oncol 1998 20: 315–318
O'Reilly S, Rowinsky E, Slichenmyer W et al. Phase I and pharmacologic studies of topotecan in patients with impaired hepatic function J Natl Cancer Inst 1996 88: 817–824
O'Reilly S, Rowinsky E, Slichenmyer W et al. Phase I and pharmacologic studies of topotecan in patients with impaired renal function J Clin Oncol 1996 14: 3062–3073
Park JR, Slattery J, Gooley T et al. Phase I topotecan preparative regimen for high-risk neuroblastoma, high-grade glioma, and refractory/recurrent pediatric solid tumors Med Pediatr Oncol 2000 35: 719–723
Calvert AH, Newell DR, Gumbrell LA et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function J Clin Oncol 1989 7: 1748–1756
Brodeur GM, Pritchard J, Berthold F et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment J Clin Oncol 1993 11: 1466–1477
Kushner BH, Kramer K, Cheung N-KV . Phase II trial of the anti-G02 monoclonal antibody 3f8 and granulocyte–macrophage colony-stimulating factor for neuroblastoma J Clin Oncol (in press)
Kushner BH, Kramer K, Cheung N-KV . Phase II trial of the anti-GD2 monoclonal antibody 3Fδ and granulocyte–macrophage colony-stimulating factor for neuroblastoma J Clin Oncol (in press)
Frei E III, Teicher BA, Holden SA et al. Preclinical studies and clinical correlation of the effect of alkylating dose Cancer Res 1988 48: 6417–6423
Teicher BA, Cucchi CA, Lee JB et al. Alkylating agents: in vitro studies of cross-resistance patterns in human cell lines Cancer Res 1986 46: 4379–4388
Wolff SN, Herzig RH, Fay JW et al. High-dose N, N′, N″–triethylenethio-phosphoramide (Thiotepa) with autologous bone marrow transplantation: phase I studies Semin Oncol 1990 17: 2–6
Saarinen UM, Hovi L, Mäkipernaa A, Riikonen P . High-dose thiotepa with autologous bone marrow rescue in pediatric solid tumors Bone Marrow Transplant 1991 8: 369–376
Heidemann RL, Packer RJ, Reaman GH et al. A phase II evaluation of thiotepa in pediatric central nervous system malignancies Cancer 1993 72: 271–275
Lucidarme N, Valteau-Couanet D, Oberlin O et al. Phase II study of high-dose thiotepa and hematopoietic stem cell transplantation in children with solid tumors Bone Marrow Transplant 1998 22: 535–540
Shea TC, Flaherty M, Elias A et al. A phase I clinical and pharmacokinetic study of carboplatin and autologous bone marrow support J Clin Oncol 1989 7: 651–661
Gaynon PS, Ettinger LJ, Baum ES et al. Carboplatin in childhood brain tumors. A Children's Cancer Study Group phase II trial Cancer 1990 66: 2465–2469
Friedman HS, Krischer JP, Burger P et al. Treatment of children with progressive or recurrent brain tumors with carboplatin or iproplatin: a Pediatric Oncology Group randomized phase II study J Clin Oncol 1992 10: 249–256
Ettinger LJ, Gaynon PS, Krailo MD et al. A phase II study of carboplatin in children with recurrent or progressive solid tumors. A report from the Children's Cancer Group Cancer 1994 73: 1297–1301
van de Wall E, Beijnen JH, Rodenhuis S . High-dose chemotherapy regimens for solid tumors Cancer Treat Rev 1995 21: 105–132
Friedman HS, Kerby T, Fields S et al. Topotecan treatment of adults with primary malignant glioma Cancer 1999 85: 1160–1165
Miller AA, Hargis JB, Lilenbaum RC et al. Phase I study of topotecan and cisplatin in patients with advanced solid tumors: a Cancer and Leukemia Group B study J Clin Oncol 1994 12: 2743–2750
Rowinsky EK, Kaufman SH, Baker SD et al. Sequences of topotecan and cisplatin: phase I, pharmacologic, and in vitro studies to examine sequence dependence J Clin Oncol 1996 14: 3074–3084
Murren JR, Anderson S, Fedele J et al. Dose-escalation and pharmacodynamic study of topotecan in combination with cyclophosphamide in patients with refractory cancer J Clin Oncol 1997 15: 148–157
Saylors RL III, Stewart CF, Zamboni WC et al. Phase I study of topotecan in combination with cyclophosphamide in pediatric patients with malignant solid tumors: a Pediatric Oncology Group study J Clin Oncol 1998 16: 945–952
Kushner BH, Kramer K, Meyers PA et al. Pilot study of topotecan and high-dose cyclophosphamide for resistant pediatric solid tumors Med Pediatr Oncol 2000 35: 468–474
Motzer RJ, Bosl GJ . High-dose chemotherapy for resistant germ cell tumors: recent advances and future directions J Natl Cancer Inst 1992 22: 1703–1709
Boulad F, Kernan NA, LaQuaglia MP et al. High-dose induction chemoradiotherapy followed by autologous bone marrow transplantation as consolidation therapy in rhabdomyosarcoma, extraosseous Ewing's sarcoma, and undifferentiated sarcoma J Clin Oncol 1998 16: 1697–1706
Kramer K, Kushner BH, Heller G, Cheung NKV . Neuroblastoma metastatic to the central nervous system: the Memorial Sloan-Kettering Cancer Center experience and a literature review Cancer 2001 91: 1510–1519
Acknowledgements
This work was supported in part by grants from the National Cancer Institute (CA61017, CA72868), Bethesda, MD; the Robert Steel Foundation, New York, NY; the Katie?s Find A Cure Fund, New York, NY; and the Justin Zahn Fund, New York, NY.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kushner, B., Cheung, NK., Kramer, K. et al. Topotecan combined with myeloablative doses of thiotepa and carboplatin for neuroblastoma, brain tumors, and other poor-risk solid tumors in children and young adults. Bone Marrow Transplant 28, 551–556 (2001). https://doi.org/10.1038/sj.bmt.1703213
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703213
Keywords
This article is cited by
-
Tandem high-dose chemotherapy with topotecan–thiotepa–carboplatin and melphalan–etoposide–carboplatin regimens for pediatric high-risk brain tumors
International Journal of Clinical Oncology (2019)
-
All-trans retinoic acid synergizes with topotecan to suppress AML cells via promoting RARα-mediated DNA damage
BMC Cancer (2016)
-
Results of induction chemotherapy in children older than 18 months with stage-4 neuroblastoma treated with an adaptive-to-response modified N7 protocol (mN7)
Clinical and Translational Oncology (2015)
-
A phase I/II study of CY and topotecan in patients with high-risk malignancies undergoing autologous hematopoietic cell transplantation: the St Jude long-term follow-up
Bone Marrow Transplantation (2012)
-
Topotecan in combination with radiotherapy in unresectable glioblastoma: a phase 2 study
Journal of Neuro-Oncology (2009)