Abstract
From 1987 to 1998, 19 of 416 patients (4.6%) who underwent autologous hematopoietic stem cell transplantation experienced peri-engraftment (within 5 days of neutrophil recovery) respiratory distress syndrome (PERDS) not attributable to infection, fluid overload, or cardiac dysfunction. The median time from stem cell infusion to onset of PERDS was 11 days (range 4–25). Risk of PERDS or its outcome was not predicted by any pre- or peri-transplant clinical or laboratory feature. The respective median white blood cell and platelet counts at first symptoms were 1.3 × 109/l and 25 × 109/l. No patients had an infectious etiology by bronchoalveolar lavage. Six of the 19 patients had alveolar hemorrhage, which was significantly correlated with high neutrophil count. PERDS was directly implicated in four deaths (21%). Eleven patients received high-dose corticosteroid therapy, including five of the six who required mechanical ventilation. Ten of these patients experienced clinical improvement, which occurred within 24 h in five. The rapid response to corticosteroid treatment and the fact that such therapy was delayed until after intubation in all the mechanically ventilated cases point to a therapeutic benefit. Bone Marrow Transplantation (2001) 27, 1299–1303.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lee CK, Gingrich RD, Hohl RJ, Ajram KA . Engraftment syndrome in autologous bone marrow and peripheral stem cell transplantation Bone Marrow Transplant 1995 16: 175–182
Cahill RA, Spitzer TR, Mazumder A . Marrow engraftment and clinical manifestations of capillary leak syndrome Bone Marrow Transplant 1996 18: 177–184
Edenfield WJ, Moores LK, Goodwin G, Lee N . An engraftment syndrome in autologous stem cell transplantation related to mononuclear cell dose Bone Marrow Transplant 2000 25: 405–409
Kawano C, Muroi K, Kuribara R et al. Engraftment syndrome after autologous peripheral blood stem cell transplantation with high numbers of peripheral blood stem cells followed by granulocyte colony-stimulating factor administration (letter) Bone Marrow Transplant 2000 25: 228–229
Marin D, Berrade J, Ferra C et al. Engraftment syndrome and survival after respiratory failure post-bone marrow transplantation Intensive Care Med 1998 24: 732–735
Moreb JS, Kubilis PS, Mullins DL et al. Increased frequency of autoaggression syndrome associated with autologous stem cell transplantation in breast cancer patients Bone Marrow Transplant 1997 19: 101–106
Ravenel JG, Scalzetti EM, Zamkoff KW . Chest radiographic features of engraftment syndrome J Thorac Imaging 2000 15: 56–60
Wright DG, Meierovics AI, Foxley JM . Assessing the delivery of neutrophils to tissues in neutropenia Blood 1986 67: 1023–1030
Krowka MJ, Rosenow EC III, Hoagland HC . Pulmonary complications of bone marrow transplantation Chest 1985 87: 237–246
Afessa B, Tefferi A, Hoagland HC et al. Outcome of recipients of bone marrow transplants who require intensive-care unit support Mayo Clin Proc 1992 67: 117–122
Paz HL, Crilley P, Weinar M, Brodsky I . Outcome of patients requiring medical ICU admission following bone marrow transplantation Chest 1993 104: 527–531
Faber-Langendoen K, Caplan AL, McGlave PB . Survival of adult bone marrow transplant patients receiving mechanical ventilation: a case for restricted use Bone Marrow Transplant 1993 12: 501–507
Rubenfeld GD, Crawford SW . Withdrawing life support from mechanically ventilated recipients of bone marrow transplants: a case for evidence-based guidelines Ann Intern Med 1996 125: 625–633
Raptis A, Mavroudis D, Suffredini A et al. High-dose corticosteroid therapy for diffuse alveolar hemorrhage in allogeneic bone marrow stem cell transplant recipients Bone Marrow Transplant 1999 24: 879–883
Metcalf JP, Rennard SI, Reed EC et al. Corticosteroids as adjunctive therapy for diffuse alveolar hemorrhage associated with bone marrow transplantation. University of Nebraska Medical Center Bone Marrow Transplant Group Am J Med 1994 96: 327–334
Chao NJ, Duncan SR, Long GD et al. Corticosteroid therapy for diffuse alveolar hemorrhage in autologous bone marrow transplant recipients Ann Intern Med 1991 114: 145–146
Ravoet C, Feremans W, Husson B et al. Clinical evidence for an engraftment syndrome associated with early and steep neutrophil recovery after autologous blood stem cell transplantation Bone Marrow Transplant 1996 18: 943–947
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Capizzi, S., Kumar, S., Huneke, N. et al. Peri-engraftment respiratory distress syndrome during autologous hematopoietic stem cell transplantation. Bone Marrow Transplant 27, 1299–1303 (2001). https://doi.org/10.1038/sj.bmt.1703075
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703075
Keywords
This article is cited by
-
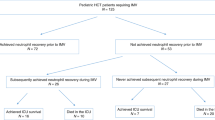
Pulmonary function and long-term survival in patients with PERDS after autologous hematopoietic stem cell transplantation
Bone Marrow Transplantation (2023)
-
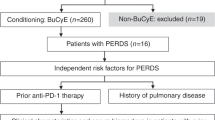
Prior anti-PD-1 therapy as a risk factor for life-threatening peri-engraftment respiratory distress syndrome in patients undergoing autologous stem cell transplantation
Bone Marrow Transplantation (2021)
-
Engraftment Syndrome: Clinical Features and Predictive Factors in Autologous Stem Cell Transplant
Indian Journal of Hematology and Blood Transfusion (2018)