Abstract
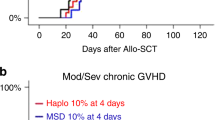
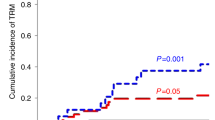
Adult T cell leukemia/lymphoma (ATL) is a poor prognosis T cell malignancy. In order to improve the outcome, we employed allogeneic stem cell transplantation (allo-SCT) for ATL in 10 patients, nine of whom were from HLA-identical siblings and one from an unrelated donor. Conditioning regimens varied among the patients except that all received total body irradiation. The patients tolerated the regimens well with mild, if any toxicity, and engraftment occurred in all cases. Median leukemia-free survival after allo-SCT was 17.5+ months (range 3.7–34.4+). Six of the 10 patients developed acute GVHD (one case each with grade I, III or IV, and three cases with grade II) and three patients developed extensive chronic GVHD. Four patients died after allo-SCT during the study period from either acute GVHD (grade IV), pneumonitis, gastrointestinal bleeding or renal insufficiency. Two of the 10 cases with no symptoms of GVHD relapsed with clinical ATL. These results strongly suggest that allo-SCT may improve the survival in ATL if a controlled degree of GVHD develops. Bone Marrow Transplantation (2001) 27, 15–20.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Uchiyama T, Yodoi J, Sagawa K et al. Adult T-cell leukemia: clinical and hematological features of 16 cases Blood 1977 50: 481–492
Poiesz BJ, Ruscetti FW, Gazdar AF et al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma Proc Natl Acad Sci USA 1980 77: 7415–7419
Hinuma Y, Nagata K, Hanaoka M et al. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera Proc Natl Acad Sci USA 1981 78: 6476–6480
Yoshida M, Miyoshi I, Hinuma Y . Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease Proc Natl Acad Sci USA 1982 79: 2031–2035
Shimoyama M, Ota K, Kikuchi M et al. Major prognostic factors of adult patients with advanced T-cell lymphoma/leukemia J Clin Oncol 1988 6: 1088–1097
Gill PS, Harrington WJ, Kaplan MH et al. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine New Engl J Med 1995 332: 1744–1748
Taguchi H, Kinoshita K, Takatsuki K et al. An intensive chemotherapy of adult T-cell leukemia/lymphoma: CHOP followed by etoposide, vindesine, ranimustine, and mitoxantrone with granulocyte colony-stimulating factor support J Acquir Immune Defic Syndr Hum Retrovirol 1996 12: 182–186
Hanada S, Utsunomiya A, Suzuki S et al. Treatment for adult T-cell leukemia Cancer Chemother Pharmacol 1997 40: (Suppl) S47–S50
Shimoyama M . The Lymphoma Study Group: diagnostic criteria and classification of clinical subtypes of adult T-cell leukemia-lymphoma Br J Haematol 1991 79: 428–437
Kato S, Nishimura J, Muta K et al. Overexpression of P-glycoprotein in adult T-cell leukemia Lancet 1990 336: 573
Kuwazuru Y, Hanada S, Furukawa T et al. Expression of P-glycoprotein in adult T-cell leukemia cells Blood 1992 76: 2065–2071
Ikeda K, Oka M, Yamada Y et al. Adult T-cell leukemia cells over-express the multidrug-resistance-protein (MRP) and lung-resistance-protein (LRP) genes Int J Cancer 1999 82: 599–604
Sobue R, Yamauchim T, Miyamura K et al. Treatment of adult T cell leukemia with mega-dose cyclophosphamide and total body irradiation followed by allogeneic bone marrow transplantation Bone Marrow Transplant 1987 2: 441–444
Ljungman P, Lawler M, Asj B et al. Infection of donor lymphocytes with human T lymphotrophic virus type 1 (HTLV-I) following allogeneic bone marrow transplantation for HTLV-I positive adult T-cell leukaemia Br J Haematol 1994 88: 403–405
Borg A, Liu Yin JA, Johnson PRE et al. Successful treatment of HTLV-1-associated adult T-cell leukaemia lymphoma by allogeneic bone marrow transplantation Br J Haematol 1996 94: 713–715
Wittwer CT, Herrmann MG, Moss AA, Rasmussen RP . Continuous fluorescence monitoring of rapid cycle DNA amplification Biotechniques 1997 22: 130–138
Kaplan E, Meier P . Nonparametric estimations from incomplete observations Am Stat Assoc J 1958 53: 457–481
Kreitman RJ, Wilson WH, White JD et al. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38(LMB-2) in patients with hematologic malignancies J Clin Oncol 2000 18: 1622–1636
Tsukasaki K, Maeda T, Arimura K et al. Poor outcome of autologous stem cell transplantation for adult T cell leukemia/lymphoma: a case report and review of the literature Bone Marrow Transplant 1999 23: 87–89
Kawa K, Nishiuchi R, Okamura T et al. Eradication of human T-lymphotropic virus type 1 by allogeneic bone-marrow transplantation Lancet 1998 352: 1034–1035
Acknowledgements
We would like to thank Dr I Sanada (Institute for Clinical Research, Kumamoto National Hospital), Dr E Ohtsuka (Second Department of Internal Medcine, Oita Medical University), Dr E Ohno (Department of Hematology, Oita Prefectural Hospital), Dr Y Nawa (Division of Internal Medicine and Hematology, Ehime Prefectural Central Hospital), Dr H Taji (Department of Hematology and Chemotherapy, Aichi Cancer Center) and Dr Y Atsuta (Division of Hematology, Japanese Red Cross Nagoya First Hospital) for providing information on ATL patients who received allo-SCT.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Utsunomiya, A., Miyazaki, Y., Takatsuka, Y. et al. Improved outcome of adult T cell leukemia/lymphoma with allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 27, 15–20 (2001). https://doi.org/10.1038/sj.bmt.1702731
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702731
Keywords
This article is cited by
-
Cord blood is a suitable donor source of allogeneic hematopoietic cell transplantation for adult T-cell leukemia-lymphoma: a nationwide retrospective study
Bone Marrow Transplantation (2023)
-
Therapeutic approaches for HTLV-1-associated adult T-cell leukemia/lymphoma: a comprehensive review
Medical Oncology (2023)
-
Mogamulizumab for post-transplant relapse of adult T-cell leukemia/lymphoma: a case study
International Journal of Hematology (2023)
-
Treatment of Adult T-Cell Leukemia/Lymphoma: Established Paradigms and Emerging Directions
Current Treatment Options in Oncology (2023)
-
Outcome of Stem Cell Transplantation in HTLV-1-Associated North American Adult T-Cell Leukemia/Lymphoma
Clinical Hematology International (2023)