Abstract
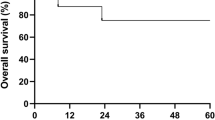
Chronic granulomatous disease (CGD) is a primary immunodeficiency disorder characterized by impaired microbial killing and susceptibility to bacterial and fungal infections. Cure of the disease can be achieved by stem cell transplantation when performed early in its course, and before severe infections have developed. Invasive aspergillosis constitutes a very high risk for transplantation. We report a 4-year-old boy with X-linked CGD who underwent successful HLA-identical peripheral blood stem cell (PBSC) transplantation during invasive pulmonary aspergillosis and osteomyelitis of the left fourth rib, which was unresponsive to antifungal treatment. During the 2 months prior to the transplant he received G-CSF-mobilized granulocyte transfusions (GTX) from unrelated donors three times a week in addition to the antifungal treatment. This resulted in clinical improvement in his respiratory status. He also received GTX during the aplastic period after the conditioning regimen, until he had engrafted. Post-transplant superoxide generation test revealed that neutrophil function was within normal range. One year post transplant the CT scan showed almost complete clearance of the pulmonary infiltrates and a marked improvement in the osteomyelitic process. Based on other reports and our own experience, GTX can serve as important treatment in patients with CGD who have failed conventional anti-fungal treatment and for whom stem cell transplantation is the only chance for cure. Bone Marrow Transplantation (2000) 26, 1025–1028.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Mouy R, Fischer A, Vilmer E et al. Incidence, severity, and prevention of infections in chronic granulomatous disease J Pediatr 1989 114: 555–560
Fischer A, Segal AW, Seger R, Weening RS . The management of chronic granulomatous disease Eur J Pediatr 1993 152: 896–899
Mouy R, Veber F, Blanche S et al. Long-term itraconazole prophylaxis against aspergillus infections in thirty-two patients with chronic granulomatous disease J Pediatr 1994 125: 998–1003
Rappeport JM, Newburger PE, Goldblum RM et al. Allogeneic bone marrow transplantation for chronic granulomatous disease J Pediatr 1982 101: 952–955
Kamani N, August CS, Douglas SD et al. Bone marrow transplantation in chronic granulomatous disease J Pediatr 1984 105: 42–46
di Bartolomeo P, di Girolamo G, Angrilli F et al. Reconstitution of normal neutrophil function in chronic granulomatous disease by bone marrow transplantation Bone Marrow Transplant 1989 4: 695–700
Hobbs JR, Monteil M, McClusey DR et al. Chronic grnaulomatous disease 100% corrected by displacement bone marrow transplantation from a volunteer unrelated donor Eur J Pediatr 1992 151: 806–810
Calvino MC, Maldonado MS, Otheo E et al. Bone marrow transplantation in chronic granulomatous disease Eur J Pediatr 1996 155: 877–879
Ho CM, Vowels MR, Lockwood L, Ziegler JB . Successful bone marrow transplantation in a child with X-linked chronic granulomatous disease Bone Marrow Transplant 1996 18: 213–215
Oszhim H, von Planta M, Muller I et al. Successful treatment of invasive aspergillosis in chronic granulomatous disease by bone marrow transplantation, granulocyte colony-stimulating factor-mobilized granulocytes, and liposomal amphotericin-B Blood 1998 92: 2719–2724
Nagler A, Ackerstein A, Kapelushnik J et al. Donor lymphocyte infusion post-non-myeloblative allogeneic peripheral blood stem cell transplantation for chronic granulomatous disease Bone Marrow Transplant 1999 24: 339–342
Bensinger WI, Price TH, Dale DC et al. The effects of daily recombinant human granulocyte colony-stimulating factor administration on normal granulocyte donors undergoing leukapheresis Blood 1993 81: 1883–1888
Buescher ES, Gallin JI . Leukocyte transfusion in chronic granulomatous disease: persistence of transfused leukocytes in sputum New Engl J Med 1982 307: 800–803
Bujak JS, Kwon-chung KJ, Chusid MJ . Osteomyelitis and pneumonia in a boy with chronic granulomatous disease of childhood caused by a mutant strain of aspergillus nidulans Am J Clin Pathol 1974 61: 361–367
Yomtovian R, Abramson J, Quie P, McCullough J . Granulocyte transfusion therapy in chronic granulomatous disease Transfusion 1981 21: 739–743
Emmendorffer A, Lohmann-Mathes ML, Roesier J . Kinetics of transfused neutrophils in peripheral blood and BAL fluid of patient with variatn X-linked chronic granulomatous disease Eur J Haematol 1991 47: 246–252
Stroneck DF, Leonard K, Eiber G et al. Alloimmunization after granulocyte transfusions Transfusion 1996 36: 1009–1015
Strauss RG . Rebirth of granulocyte transfusion: should it involve pediatric oncology and transplant patients? J Pediatr Hematol Oncol 1999 21: 475–478
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bielorai, B., Toren, A., Wolach, B. et al. Successful treatment of invasive aspergillosis in chronic granulomatous disease by granulocyte transfusions followed by peripheral blood stem cell transplantation. Bone Marrow Transplant 26, 1025–1028 (2000). https://doi.org/10.1038/sj.bmt.1702651
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702651
Keywords
This article is cited by
-
Granulocyte Transfusions in Patients with Chronic Granulomatous Disease Undergoing Hematopoietic Cell Transplantation or Gene Therapy
Journal of Clinical Immunology (2022)
-
Chronic Granulomatous Disease: a Comprehensive Review
Clinical Reviews in Allergy & Immunology (2021)
-
Stem cell applications in military medicine
Stem Cell Research & Therapy (2011)
-
Treosulfan-based conditioning regimen in a second matched unrelated peripheral blood stem cell transplantation for a pediatric patient with CGD and invasive aspergillosis, who experienced initial graft failure after RIC
International Journal of Hematology (2009)