Abstract
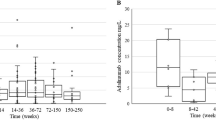
Cyclosporin A (CsA) absorption is highly variable in BMT patients. Neoral, a new microemulsion formulation of CsA, permits increased absorption with less variable pharmacokinetic parameters in non-BMT patients. We evaluated the pharmacokinetics of CsA after BMT in patients received microemulsion CsA. Two oral doses of 3 mg/kg were given 48 h apart between 14 and 28 days after allogeneic BMT in 20 adults, and one dose in seven children, while subjects were receiving a continuous i.v. infusion of CsA. Whole blood samples were taken throughout the dosing interval to calculate the incremental CsA exposure using maximum concentration (Cmax), time to Cmax (tmax), concentration at 12 h after the dose (C12), the area under the concentration-time curve (AUC), and to establish inter- and intra-patient pharmacokinetic variability. Drug exposure was substantially lower in children than in adults, with an AUC of 861 ± 805 vs2629 ± 1487 μmg × h/l (P = 0.001), respectively, and absorption was delayed and diminished in both groups by comparison with solid organ recipients. Intra-patient variability in adults for AUC was high at 0.59 ± 0.34, while inter-patient variability, measured as the coefficient of variation (c.v.), was 0.55 for the first and 0.54 for the second dose. In adults, gastrointestinal (GI) inflammation due to either mucositis or GVHD resulted in a higher AUC of 3077 ± 1551 μg × h/l compared to 1795 ± 973 μg × h/l (P = 0.02), and a similar trend was observed in children. AUC seemed little affected by the CsA formulation (liquid or capsule), or co-administration with liquids or food. Trough (12 h) CsA levels correlated poorly with incremental AUC. Sparse sample modeling of the AUC using two-point predictors taken at 2.5 and 5 h after dosing accurately approximated AUC in adults (r2 = 0.94), while 1.5 and 5 h was superior in children (r2 = 0.98). These data suggest that 12 h post-dose trough measurements of CsA may not be the most appropriate way to evaluate CsA blood concentrations in order to establish therapeutic efficacy in BMT patients. Based on this study, the dose of microemulsion CsA should be adjusted based on recipient age, and the presence of GI inflammation secondary to mucositis or GVHD. These data would suggest that sparse sampling at time points earlier than the trough more accurately reflects the AUC and may correlate more closely with therapeutic efficacy early post-BMT. Bone Marrow Transplantation (2000) 26, 545–551.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ptachcinski RJ, Venkataramanan R, Burkart GJ . Clinical pharmacokinetics of cyclosporin Clin Pharmacokinet 1986 11: 107–132
Kahan BD . Individualization of cyclosporine therapy using pharmacokinetic and pharmacodynamic parameters Transplantation 1985 40: 457–476
Yee GC . Recent advances in cyclosporine pharmacokinetics Pharmacotherapy 1991 11: 130S-134S
Shaw LM, Bowers L, Demers L et al. Critical issues in cyclosporine monitoring: report of the task force on cyclosporine monitoring Clin Chem 1987 33: 1269–1288
Fahr A . Cyclosporin clinical pharmacokinetics Clin Pharmacokinet 1993 24: 472–495
Lindholm A . Factors influencing the pharmacokinetics of cyclosporine in man Ther Drug Monit 1991 13: 465–477
Lindholm A, Henricsson S, Lind M et al. Intraindividual variability in the relative systemic availability of cyclosporine after oral dosing Eur J Clin Pharmacol 1988 34: 46–44
Sandimmune Neoral product monograph Sandoz Canada Inc: Quebec, 11 January 1995
Superina RA, Strong DK, Acal LA, DeLuca E . Relative bioavailability of Sandimmune and Sandimmune Neoral in pediatric liver recipients Transplant Proc 1994 26: 2979–2980
McDonald GB, Shulman HM, Sullivan KM, Spencer GD . Intestinal and hepatic complications of human bone marrow transplantation. Part II Gastroenterology 1986 90: 770–784
Atkinson K, Biggs JC, Britton R et al. Oral administration of cyclosporin A for recipients of allogeneic marrow transplants: implications of clinical gut dysfunction Br J Haematol 1984 56: 223–231
Miller AB, Hoogstraten B, Staquet M, Winkler A . Reporting results of cancer treatment Cancer 1981 47: 207–214
Cooney GF, Habucky K, Hoppu K . Cyclosporin pharmacokinetics in paediatric transplant recipients Clin Pharmacokinet 1997 32: 481–495
Kabasakul SC, Clarke M, Kane H et al. Comparison of Neoral and Sandimmun cyclosporin A pharmacokinetic profiles in young renal transplant recipients Pediatr Nephrol 1997 11: 318–321
Melter M, Rodeck B, Kardorff R et al. Pharmacokinetics of cyclosporine in pediatric long-term liver transplant recipients converted from Sandimmun to Neoral Transpl Int 1997 10: 419–425
Lee YJ, Chung SJ, Shim CK . Decreased oral availability of cyclosporin A at second administration in humans Br J Clin Pharmacol 1997 44: 343–345
Krmar RT, Wuhl E, Ding R et al. Pharmacokinetics of a new microemulsion formulation of cyclosporin A (Neoral) in young patients after renal transplantation Transpl Int 1996 9: 476–480
Fricker G, Drewe J, Huwyler J et al. Relevance of P-glycoprotein for the enteral absorption of cyclosporin A: in vitro-in vivo correlation Br J Pharmacol 1996 118: 1841–1847
Lown KS, Mayo RR, Leichtman AB et al. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine Clin Pharmacol Ther 1997 62: 248–260
Schwinghammer TL, Przepiorka D, Venkataramanan R et al. The kinetics of cyclosporine and its metabolites in bone marrow transplant patients Br J Clin Pharmacol 1991 32: 323–328
Augustijns PF, Bradshaw TP, Gan LS et al. Evidence for a polarized efflux system in CACO-2 cells capable of modulating cyclosporin A transport Biochem Biophys Res Comm 1993 197: 360–365
Hunter J, Hirst BH . Intestinal secretion of drugs – the role of P-glycoprotein and related drug efflux systems in limiting oral drug absorption Adv Drug Del Rev 1997 25: 129–157
Gan LSL, Moseley MA, Khosla B et al. Cyp3a-like cytochrome P450-mediated metabolism and polarized efflux of cyclosporin A in Caco-2 cells – interaction between the two biochemical barriers to intestinal transport Drug Metab Dispos 1996 24: 344–349
Kivisto KT, Kroemer HK, Eichelbaum M . The role of human cytochrome P450 enzymes in the metabolism of anticancer agents – implications for drug interactions Br J Clin Pharmacol 1995 40: 523–530
Fuhr U . Drug interactions with grapefruit juice – extent, probable mechanism and clinical relevance Drug Safety 1998 18: 251–272
Bandini G, Strocchi E, Ricci P et al. Cyclosporin A: correlation of blood levels with acute graft-versus-host disease after bone marrow transplantation Acta Haematol 1987 78: 6–12
Vogelsang GB, Morris LE . Prevention and management of graft-versus-host disease. Practical recommendations Drugs 1993 45: 668–676
Yee GC, Lennon TP, Gmur DJ et al. Age-dependent cyclosporine: pharmacokinetics in marrow transplant recipients Clin Pharmacol Ther 1986 40: 438–443
Campana C, Regazzi MB, Buggia I, Molinaro M . Clinically significant drug interactions with cyclosporin – an update Clin Pharmacokinet 1996 30: 141–179
Acknowledgements
We wish to thank the BMT nurses and physicians in the BMT programs and Julia Schultz for editorial assistance in preparation of this manuscript. In particular, we would like to thank Drs Ron Anderson, Jeff Davis, Chris Fryer, Sheila Pritchard, John Sheperd, Stephen Nantel, Hans-G Klingemann, Michael Barnett, Donna Hogge, Heather Sutherland, and John Wu. This study was fully funded by an unrestricted grant from Novartis Pharma Canada Inc (Dorval, Canada). Part of the data presented in this manuscript has been published in Transplant Proc 1998; 30: 1668–1670.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schultz, K., Nevill, T., Balshaw, R. et al. Effect of gastrointestinal inflammation and age on the pharmacokinetics of oral microemulsion cyclosporin A in the first month after bone marrow transplantation. Bone Marrow Transplant 26, 545–551 (2000). https://doi.org/10.1038/sj.bmt.1702545
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702545
Keywords
This article is cited by
-
A decision support tool to find the best cyclosporine dose when switching from intravenous to oral route in pediatric stem cell transplant patients
European Journal of Clinical Pharmacology (2020)
-
Converting cyclosporine A from intravenous to oral administration in hematopoietic stem cell transplant recipients and the role of azole antifungals
European Journal of Clinical Pharmacology (2018)
-
Bayesian Networks: A New Approach to Predict Therapeutic Range Achievement of Initial Cyclosporine Blood Concentration After Pediatric Hematopoietic Stem Cell Transplantation
Drugs in R&D (2018)
-
Pharmacokinetics, Pharmacodynamics and Pharmacogenomics of Immunosuppressants in Allogeneic Haematopoietic Cell Transplantation: Part I
Clinical Pharmacokinetics (2016)
-
Bayesian approach for the estimation of cyclosporine area under the curve using limited sampling strategies in pediatric hematopoietic stem cell transplantation
Theoretical Biology and Medical Modelling (2014)