Abstract
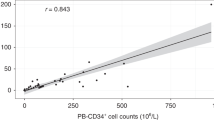
Leukapheresis collections obtained following one of four mobilization regimens from 90 cancer patients were analyzed for their content of various progenitor cell types including erythroid and granulopoietic colony-forming cells in methylcellulose (total CFC), CFC-megakaryocyte (CFC-Mk), CFC detected after 10, 35 and 56 days in long-term culture (LTC), and total CD34+ cells. The number of each of these progenitor cell types collected from individual patients varied over 1000-fold. Nevertheless, within an individual leukapheresis, there was a significant correlation between the number of CD34+ cells and each progenitor type (except day 56 LTC CFC) suggesting that all of them are mobilized by a common mechanism. Patients who had previously received extensive chemotherapy and/or radiotherapy mobilized fewer of all these cell types than those who had not. For the 65 patients who proceeded to autologous transplantation, the median times to an absolute neutrophil count (ANC) of ⩾0.5 × 109/l and the last platelet transfusion post transplant were 13 and 11 days, respectively, with 14 (22%) of patients having platelet recovery delayed beyond day 21. There was no significant difference between patients who had or had not received extensive chemo/radiotherapy or among the different mobilization regimens for time to neutrophil or platelet recovery or the number of platelet or red blood cell transfusions received post transplant. Threshold doses of the different cell types transplanted (per kg of patient weight) which predicted rapid platelet recovery were 2 × 106 CD34+ cells, 5 × 105 total CFC and 2.5 × 104CFC-Mk. Corresponding thresholds for progenitor activity measured in LTC could not be established. These results further support the view that standard mobilization regimens yield progenitor numbers that are, in most cases, nonlimiting for generating neutrophil and platelet recoveries within 2 to 3 weeks after myeloablative therapy. Assessment of the CD34+ cell and/or CFC content of leukapheresis collections may identify patients in whom platelet recovery is likely to be significantly delayed although CFC-Mk enumeration does not appear to offer any unique predictive advantage. Bone Marrow Transplantation (2000) 25, 589–598.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Sheridan WP, Begley CG, Juttner CA et al. Effect of peripheral-blood progenitor cells mobilised by filgrastim (G-CSF) on platelet recovery after high-dose chemotherapy Lancet 1992 339: 640–644
Juttner CA, To LB, Ho JQK et al. Early lympho-hemopoietic recovery after autografting using peripheral blood stem cells in acute non-lymphoblastic leukemia Transplant Proc 1988 20: 40–43
Korbling M, Fliedner TM, Holle R et al. Autologous blood stem cell (ABSCT) vs purged bone marrow transplantation (pABMT) in standard risk AML: Influence of source and cell composition of the autograft on hemopoietic cell survival Bone Marrow Transplant 1991 7: 343–349
Gianni AM, Siena S, Bregni M et al. Granulocyte–macrophage colony-stimulating factor to harvest circulating haemopoietic stem cells for autotransplantation Lancet 1989 2: 580–584
Socinski MA, Cannistra SA, Elias A et al. Granulocyte–macrophage colony stimulating factor expands the circulating haemopoietic progenitor cell compartment in man Lancet 1988 1: 1194–1198
Sutherland HJ, Eaves CJ, Lansdorp PM et al. Kinetics of committed and primitive blood progenitor mobilization after chemotherapy and growth factor treatment and their use in autotransplants Blood 1994 83: 3808–3814
Chao NJ, Schriber JR, Grimes K et al. Granulocyte colony-stimulating factor ‘mobilized’ peripheral blood progenitor cells accelerate granulocyte and platelet recovery after high-dose chemotherapy Blood 1993 81: 2031–2035
Bishop MR, Anderson JR, Jackson JD et al. High-dose therapy and peripheral blood progenitor cell transplantation: effects of recombinant human granulocyte–macrophage colony-stimulating factor on the autografts Blood 1994 83: 610–616
Briddell RA, Hartley CA, Smith KA, McNiece IK . Recombinant rat stem cell factor synergizes with recombinant human granulocyte colony-stimulating factor in vivo in mice to mobilize peripheral blood progenitor cells that have enhanced repopulating potential Blood 1993 82: 1720–1723
Brugger W, Bross K, Frisch J et al. Mobilization of peripheral blood progenitor cells by sequential administration of interleukin-3 and granulocyte–macrophage colony-stimulating factor following polychemotherapy with cisplatin Blood 1992 79: 1193–1200
Siena S, Bregni M, Bonsi L et al. Increase in peripheral blood megakaryocyte progenitors following cancer therapy with high-dose cyclophosphamide and hematopoietic growth factors Exp Hematol 1993 21: 1583–1590
Begley CG, Basser R, Mansfield R et al. Enhanced levels and enhanced clonogenic capacity of blood progenitor cells following administration of stem cell factor plus granulocyte colony-stimulating factor to humans Blood 1997 90: 3378–3389
Lie AKW, Rawling TP, Bayly JL, To LB . Progenitor cell yield in sequential blood stem cell mobilization in the same patients: insights into chemotherapy dose escalation and combination of haemopoietic growth factor and chemotherapy Br J Haematol 1996 95: 39–44
Siena S, Bregni M, Brando B et al. Circulation of CD34+ hematopoietic stem cells in the peripheral blood of high-dose cyclophosphamide-treated patients: enhancement by intravenous recombinant human granulocyte–macrophage colony-stimulating factor Blood 1989 74: 1905–1914
Tanaka R, Matsudaira T, Aizawa J et al. Characterization of peripheral blood progenitor cells (PBPC) mobilized by filgrastim (rHuG-CSF) in normal volunteers: dose–effect relationship for filgrastim with the character of mobilized PBPC Br J Haematol 1996 92: 795–803
Drake M, Ranaghan L, Morris TCM et al. Analysis of the effect of prior therapy on progenitor cell yield: use of a chemotherapy scoring system Br J Haematol 1997 98: 745–749
Neben S, Hemman S, Montgomery M et al. Hematopoietic stem cell deficit of transplanted bone marrow previously exposed to cytotoxic agents Exp Hematol 1993 21: 156–162
Haas R, Mohle R, Fruhauf S et al. Patient characteristics associated with successful mobilizing and autografting of peripheral blood progenitor cells in malignant lymphoma Blood 1994 83: 3787–3794
Hogge D, Fanning S, Bockhold K et al. Quantitation and characterization of human megakaryocyte colony-forming cells using a standardized serum-free agarose assay Br J Haematol 1997 96: 790–800
Sutherland HJ, Lansdorp PM, Henkelman DH et al. Functional characterization of individual human hematopoietic stem cells cultured at limiting dilution on supportive marrow stromal layers Proc Natl Acad Sci USA 1990 87: 3584–3588
Sutherland DR, Keating A, Nayar R et al. Sensitive detection and enumeration of CD34+ cells in peripheral and cord blood by flow cytometry Exp Hematol 1994 22: 1003–1010
Prince HM, Imrie K, Keating A et al. The influence of prior alkylating agent therapy on the collection of peripheral blood progenitor cells in multiple myeloma Exp Hematol 1995 23: 755 (Abstr.)
Morton J, Morton A, Bird R et al. Predictors for optimal mobilization and subsequent engraftment of peripheral blood progenitor cells following intermediate dose cyclophosphamide and G-CSF Leukemia Res 1997 21: 21–27
Reece DE, Barnett MJ, Shepherd JD et al. High-dose cyclophosphamide, BCNU, and VP16–213 with or without cisplatin (CBV±P) and autologous transplantation for patients with Hodgkin's disease who fail to enter a complete remission after combination chemotherapy Blood 1995 86: 451–456
Phillips GL, Shepherd JD, Barnett MJ et al. Busulfan, cyclophosphamide, and melphalan conditioning for autologous bone marrow transplantation in hematologic malignancy J Clin Oncol 1991 9: 1880–1888
Shepherd JD, Barnett MJ, Connors JM et al. Allogeneic bone marrow transplantation for poor-prognosis non-Hodgkin's lymphoma Bone Marrow Transplant 1993 12: 591–596
Hogge DE, Lee CY, Benny WB, Sutherland HJ . Collection of peripheral blood mononuclear cells as a byproduct of platelet pheresis with two different blood cell separators J Clin Apheresis 1992 7: 208–212
Coulombel L, Eaves AC, Eaves CJ . Enzymatic treatment of long-term human marrow cultures reveals the preferential location of primitive hemopoietic progenitors in the adherent layer Blood 1983 62: 291–297
Hogge DE, Lansdorp PM, Reid D et al. Enhanced detection, maintenance and differentiation of primitive human hematopoietic cells in cultures containing murine fibroblasts engineered to produce human Steel factor, interleukin-3 and granulocyte colony-stimulating factor Blood 1996 88: 3765–3773
Eaves CJ, Cashman JD, Eaves AC . Methodology of long-term culture of human hemopoietic cells J Tissue Cult Meth 1991 13: 55–62
Udomsakdi C, Lansdorp PM, Hogge DE et al. Characterization of primitive hematopoietic cells in normal human peripheral blood Blood 1992 80: 2513–2521
Prosper F, Stroncek D, Verfaillie CM . Phenotypic and functional characterization of long-term culture-initiating cells present in peripheral blood progenitor collections of normal donors treated with granulocyte colony-stimulating factor Blood 1996 88: 2033–2042
Hao QL, Thiemann FT, Petersen D et al. Extended long-term culture reveals a highly quiescent and primitive human hematopoietic progenitor population Blood 1996 88: 3306–3313
Cox DR . Regression models and life tables J R Stat Soc (B) 1972 34: 187–220
Bensinger W, Singer J, Appelbaum F et al. Autologous transplantation with peripheral blood mononuclear cells collected after administration of recombinant granulocyte stimulating factor Blood 1993 81: 3158–3163
Dercksen MW, Weimar IS, Richel DJ et al. The value of flow cytometric analysis of platelet glycoprotein expression on CD34+ cells measured under conditions that prevent P-selectin-mediated binding of platelets Blood 1995 86: 3771–3782
Smith C, Gasparetto C, Collins N et al. Purification and partial characterization of a human hematopoietic precursor population Blood 1991 77: 2122–2128
Petzer AL, Hogge DE, Lansdorp PM et al. Self-renewal of primitive human hematopoietic cells (long-term-culture-initiating cells) in vitro and their expansion in defined medium Proc Natl Acad Sci USA 1996 93: 1470–1474
Conneally E, Cashman J, Petzer A, Eaves C . Expansion in vitro of transplantable human cord blood stem cells demonstrated using a quantitative assay of their lympho-myeloid repopulating activity in nonobese diabetic-scid/scid mice Proc Natl Acad Sci USA 1997 94: 9836–9841
Gianni AM, Siena S, Bregni M et al. Recombinant human interleukin-3 hastens trilineage hematopoietic recovery following high-dose (7g/m+) cyclophosphamide cancer therapy Ann Oncol 1993 4: 759–766
Kotasek D, Shepherd KM, Sage RE et al. Factors affecting blood stem cell collections following high-dose cyclophosphamide mobilization in lymphoma, myeloma and solid tumors Bone Marrow Transplant 1992 9: 11–17
Chatta GS, Price TH, Allen RC, Dale DC . Effects of in vivo recombinant methionyl human granulocyte colony-stimulating factor on the neutrophil response and peripheral blood colony-forming cells in healthy young and elderly adult volunteers Blood 1994 84: 2923–2929
Tong J, Hoffman R, Siena S et al. Characterization and quantitation of primitive hematopoietic progenitor cells present in peripheral blood autografts Exp Hematol 1994 22: 1016–1024
Acknowledgements
We thank Cangene, Novartis and StemCell for gifts of reagents, members of the Stem Cell Assay Service and Cryogenic Laboratory of the British Columbia Cancer Agency and the Cell Separator Unit of the Vancouver General Hospital for technical support, Alan Le for help with statistical analysis, and Christine Kelly for typing the manuscript. This work was supported by grants from the British Columbia Health Research Foundation and the National Cancer Institute of Canada (NCIC) with funds from the Terry Fox Run. CJ Eaves is a Terry Fox Cancer Research Scientist of the NCIC.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hogge, D., Lambie, K., Sutherland, H. et al. Quantitation of primitive and lineage-committed progenitors in mobilized peripheral blood for prediction of platelet recovery post autologous transplant. Bone Marrow Transplant 25, 589–598 (2000). https://doi.org/10.1038/sj.bmt.1702211
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702211
Keywords
This article is cited by
-
Short treatment of peripheral blood cells product with Fas ligand using closed automated cell processing system significantly reduces immune cell reactivity of the graft in vitro and in vivo
Bone Marrow Transplantation (2022)
-
EPO in combination with G-CSF improves mobilization effectiveness after chemotherapy with ifosfamide, epirubicin and etoposide and reduces costs during mobilization and transplantation of autologous hematopoietic progenitor cells
Bone Marrow Transplantation (2009)
-
Pegfilgrastim successfully mobilizes megakaryocyte progenitors into the peripheral blood in subjects with solid tumours
Bone Marrow Transplantation (2008)
-
Effect of peripheral blood progenitor cell dose on hematopoietic recovery: identification of minimal progenitor cell requirements for rapid engraftment
Bone Marrow Transplantation (2004)
-
Progenitor content of autologous grafts: mobilized bone marrow vs mobilized blood
Bone Marrow Transplantation (2003)