Abstract
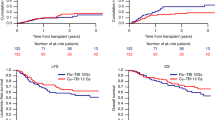
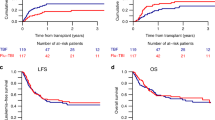
Marrow-ablative chemo-radiotherapy followed by hematopoietic stem cell rescue from an allogeneic source improves outcomes for children with high-risk acute leukemia. The first effective pre-transplant preparative regimens consisted of high-dose cyclophosphamide (CY) and total body irradiation (TBI). Subsequent attempts have been made to improve leukemia-free survival, by adding other chemotherapy agents to these agents. In previous clinical studies of total body irradiation, etoposide, cyclophosphamide (TBI-VP-16-Cy) in adult allogeneic bone marrow transplantation, there has been a high incidence of severe regimen-related toxicity. In this study, we investigated the safety and efficacy of this combination in 41 children who received TBI (12–14 Gy), VP-16 (30 mg/kg), and CY (60 mg/kg × 2) and then either matched sibling or alternative donor transplants for acute leukemia. There was only one case of fatal regimen-related toxicity. The estimated 3-year event-free survival for patients with early or intermediate stage disease was 68% (53–88%). The estimated event-free survival of patients with advanced disease was 17% (5–59%). TBI-VP16-CY is safe in pediatric transplantation, and it has good efficacy for transplant recipients with less advanced disease. Bone Marrow Transplantation (2000) 25, 489–494.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sanders JE, Thomas ED, Buckner CD et al. Marrow transplantation for children in first remission of acute non-lymphoblastic leukemia: an update Blood 1985 66: 460–462
Brochstein JA, Kernan NA, Goshen S et al. Allogeneic bone marrow transplantation after hyperfractionated total-body-irradiation and cyclophosphamide in children with acute leukemia New Engl J Med 1987 317: 1618–1624
Clift RA, Buckner CD, Applebaum FR et al. Allogeneic bone marrow transplantation in patients with AML in first remission: a randomized trial of two irradiation regimens Blood 1990 76: 1867–1871
Lynch MHE, Petersen FB, Applebaum FR et al. Phase II study of busulfan, cyclophosphamide and fractionated total body irradiation as a preparatory regimen for allogeneic bone marrow transplantation in patients with advanced myeloid malignancies Bone Marrow Transplant 1996 15: 59–64
Hongeng S, Krance RA, Bowman LC et al. Outcomes of transplantation with matched-sibling and unrelated-donor bone marrow in children with leukemia Lancet 1997 350: 767–771
Brown RA, Wolff SN, Fay JW et al. High-dose etoposide, cyclophosphamide, and total-body irradiation with allogeneic bone marrow transplantation for patients with acute myeloid leukemia in first relapse: a study by the North American Marrow Transplant Group Blood 1995 85: 1391–1395
Giralt SA, LeMaistre CF, Vriesendorp HM et al. Etoposide, cyclophosphamide, total-body irradiation and allogeneic bone marrow transplantation for hematologic malignancies J Clin Oncol 1994 12: 1923–1930
Yau JC, LeMaistre CF, Andersson BS et al. Allogeneic bone marrow transplantation for hematologic malignancies following etoposide, cyclophosphamide and total-body irradiation Am J Hematol 1992 41: 40–44
Glucksberg H, Storb R, Fefer A et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA matched sibling donors Transplantation 1974 18: 295–299
Seber A, Vogelsang GB . Chronic graft-versus-host disease. In: Barrett J, Treleaven J (eds) The Clinical Practice of Stem-Cell Transplantation Mosby-Year Book Inc: St Louis, MO 1998 p 621
Bearman SI, Applebaum FR, Buckner CD et al. Regimen-related toxicity in patients undergoing bone marrow transplantation J Clin Oncol 1988 6: 1562–1568
Szydlo R, Goldman JM, Klein JP et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings J Clin Oncol 1997 15: 1767–1777
Blume KG, Kopecky KJ, Henslee-Downey JP et al. A prospective randomized comparison of total body irradiation-etoposide versus busulfan-cyclophosphamide as preparatoryregimens for bone marrow transplantation in patients with leukemia who were not in first remission: A Southwest Oncology Group Study Blood 1993 81: 2187–2193
Amylon MD, Co JP, Snyder DS et al. Allogeneic bone marrow transplant in pediatric patients with high risk hematopoietic malignancies early in the course of their disease J Pediatr Hematol Oncol 1997 19: 54–61
Chang T, Gulati S, Chou T et al. Synergistic effect of 4-hydroxycylophosphamide and etoposide on a human promyelocytic leukemia cell line (HL-60) demonstrated by computer analysis Cancer Res 1985 45: 2234–2339
Parsons SK, Castellino SM, Lehmann LE et al. Relapsed acute lymphoblastic leukemia: similar outcomes for autologous and allogeneic marrow transplantation in selected children Bone Marrow Transplant 1996 17: 763–768
Horstmann M, Kroschke G, Stockschlader M et al. Early toxicity of intensified conditioning with etoposide combined with total body irradiation/cyclophosphamide or busulfan/cyclophosphamide in children undergoing autologous or allogeneic bone marrow transplantation Pediatr Hematol Oncol 1996 13: 45–53
McDonald GB, Hinds MS, Fisher LD et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients Ann Intern Med 1993 118: 255–267
Weiner RS, Bortin MM, Gale RP et al. Interstitial pneumonitis after bone marrow transplantation. Assessment of risk factors Ann Intern Med 1986 104: 168–175
Weiner RS, Horowitz MM, Gale RP et al. Risk factors for interstitial pneumonia following bone marrow transplantation for severe aplastic anemia Br J Haematol 1989 71: 535–543
Rozman C, Carreras E, Qian C et al. Risk factors for hepatic veno-occlusive disease following HLA-identical sibling bone marrow transplants for leukemia Bone Marrow Transplant 1996 17: 75–80
Horowitz MM, Bortin MM . Results of bone marrow transplants from human leukocyte antigen identical sibling donors for treatment of childhood leukemias: a report from the international bone marrow transplant registry Am J Pediatr Hematol/Oncol 1993 15: 56–64
Horowitz MM, Gale RP, Sondel PM et al. Graft-versus-leukemia reactions after bone marrow transplantation Blood 1990 75: 555–562
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Duerst, R., Horan, J., Liesveld, J. et al. Allogeneic bone marrow transplantation for children with acute leukemia: cytoreduction with fractionated total body irradiation, high-dose etoposide and cyclophosphamide. Bone Marrow Transplant 25, 489–494 (2000). https://doi.org/10.1038/sj.bmt.1702181
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702181
Keywords
This article is cited by
-
Allogeneic hematopoietic stem cell transplantation in adult acute lymphoblastic leukemia: potential benefit of medium-dose etoposide conditioning
Experimental Hematology & Oncology (2015)
-
Treosulfan-based conditioning regimens for allogeneic HSCT in children with acute lymphoblastic leukaemia
Annals of Hematology (2015)
-
TBI and melphalan followed by allogeneic hematopoietic SCT in children with advanced hematological malignancies
Bone Marrow Transplantation (2011)
-
Fludarabine, amsacrine, high-dose cytarabine and 12 Gy total body irradiation followed by allogeneic hematopoietic stem cell transplantation is effective in patients with relapsed or high-risk acute lymphoblastic leukemia
Bone Marrow Transplantation (2009)
-
Comparative outcome of hematopoietic stem cell transplantation for pediatric acute lymphoblastic leukemia following cyclophosphamide and total body irradiation or VP16 and total body irradiation conditioning regimens
Bone Marrow Transplantation (2006)