Abstract
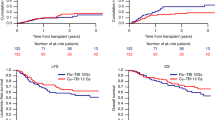
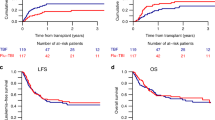
The role of more intense conditioning for second transplant was evaluated in myeloma patients achieving at least partial remission (PR) after first transplant with melphalan at 200 mg/m2. Forty-three patients received more intensive conditioning for the second transplant. Nineteen patients received cyclophosphamide 120 mg/kg along with melphalan 200 g/m2 (MEL-CY; group 1) while 24 patients received total body irradiation (1125 cGy) in conjunction with melphalan 140 mg/m2 (MEL-TBI; group 2). Forty-three matched control patients were identified from 450 patients receiving melphalan alone for second transplant (MEL200; group 3). Engraftment and toxicities were comparable among the groups with the exception of increased treatment-related mortality of 8% in group 2 compared to none in groups 1 and 3 (P = 0.07). Despite identical CR rates of 74, 71 and 70%, respectively, in groups 1, 2 and 3 (P = 1.0), event-free survival (median: 27, 15 and 61; P < 0.0001) and overall survival (median: 39, 25 and 76 months; P = 0.003) were significantly decreased in patients receiving more intensive conditioning (groups 1 and 2). Lymphocyte recovery, evaluated as a surrogate for immune recovery, was inferior in more intensively treated patients (groups 1 and 2 compared to group 3). Our findings suggest that more intense conditioning appears to have no benefit in patients responding to their first cycle of high-dose therapy and may even be detrimental in this setting. Bone Marrow Transplantation (2000) 25, 483–487.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Attal M, Harousseau JL, Stoppa AM et al. A prospective randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma New Engl J Med 1996 335: 91–96
Barlogie B, Jagannath S, Vesole DH et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma Blood 1997 89: 789–793
Ventura GJ, Barlogie B, Hester JP et al. High dose cyclophosphamide, BCNU and VP-16 with autologous blood stem cell support for refractory multiple myeloma Bone Marrow Transplant 1990 5: 265–268
Vesole DH, Tricot G, Jagannath S et al. Autotransplants in multiple myeloma; what have we learned? Blood 1996 88: 838–847
Jagannath S, Vesole DH, Glenn L et al. Low-risk intensive therapy for multiple myeloma with combined autologous bone marrow and blood stem cell support Blood 1992 80: 1666–1672
Cunningham D, Paz-Ares L, Gore M et al. High-dose melphalan for multiple myeloma; long term follow-up data J Clin Oncol 1994 12: 764–768
Cunningham D, Paz-Ares L, Milan S et al. High-dose melphalan and autologous bone marrow transplantation as consolidation in previously untreated myeloma J Clin Oncol 1994 12: 759–763
Anderson KC, Andersen J, Soiffer R et al. Monoclonal antibody-purged bone marrow transplantation therapy for multiple myeloma Blood 1993 82: 2568–2576
Marit G, Faberes C, Pico JL et al. Autologous peripheral blood progenitor cell support following high-dose chemotherapy or chemoradiotherapy in patients with high-risk multiple myeloma J Clin Oncol 1996 14: 1306–1313
Fermand JP, Chevret S, Ravaud P et al. High-dose chemoradiotherapy and autologous blood stem cell transplantation in multiple myeloma; results of a phase II trial involving 63 patients Blood 1993 82: 2005–2009
Harousseau JL, Attal M, Divine M et al. Autologous stem cell transplantation after remission induction treatment in multiple myeloma. A report of the French registry on autologous transplantation in multiple myeloma Blood 1995 85: 3077–3085
Goldschmidt H, Hegenbart U, Wallmeier M et al. Peripheral blood progenitor cell transplantation in multiple myeloma following high-dose melphalan-based therapy Rec Res Clin Cancer 1998 144: 27–35
Bjorkstrand B, Svensson H, Ljungman P et al. 2522 autotransplants in multiple myeloma. A registry study from the European group for Blood and Marrow Transplantation (EBMT) Blood 1997 90: 418a
Samlowski WE, Araneo BA, Butler MO et al. Peripheral lymph node helper T-cell recovery after syngeneic bone marrow transplantation in mice prepared with either gamma-irradiation or busulfan Blood 1989 74: 1436–1445
Bianchi A, Montacchini L, Barral P et al. CD3-induced T-cell activation in the bone marrow of myeloma patients: major role of CD4 cells Br J Haematol 1995 90: 625–632
Miguel-Garcia A, Matutes E, Tarin F et al. Circulating Ki 67 positive lymphocytes in multiple myeloma and benign monoclonal gammopathy J Clin Pathol 1995 48: 835–839
Munshi NC . Immunoregulatory mechanisms in multiple myeloma Hematol Oncol Clin North Am 1997 11: 51–69
Kay NE, Bone N, Leong T et al. Sequential phenotypic analysis of blood immune cells in myeloma patients treated on ECOG protocol E9486; relationship of immune cell to treatment and outcome Blood 1997 90: 357a
Munshi NC, Jagannath S, Desikan K et al. Significance of recovery of uninvolved immunoglobulin following total therapy in newly diagnosed multiple myeloma Blood 1997 90: 353a
Powles R, Singhal S, Treleaven J et al. Identification of patients who may benefit from prophylactic immunotherapy after bone marrow transplantation for acute myeloid leukemia on the basis of lymphocyte recovery early after transplantation Blood 1998 91: 3481–3486
Osterborg A, Yi Q, Henriksson L et al. Idiotype immunization combined with granulocyte–macrophage colony-stimulating factor in myeloma patients induced type 1, major histocompatibility complex-restricted CD8- and CD4-specific T-cell responses Blood 1998 91: 2459–2466
Kwak LW, Sternas LA, Jagannath S et al. T-cell responses elicited by immunization of multiple myeloma patients with idiotypic M-protein plus GM-CSF in remission after autologous transplantation (PSCT) Blood 1997 90: 579a
Wen YJ, Ling M, Bailey-Wood R, Lim SH . Idiotypic protein-pulsed adherent peripheral blood mononuclear cell-derived dendritic cells prime immune system in multiple myeloma Clin Cancer Res 1998 4: 957–962
Fermand JP, Ravaud P, Chevret S et al. High dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multi-center sequential randomized clinical trial Blood 1998 22: 957–963
Attal M, Payen C, Facon T et al. Single versus double transplant in myeloma; a randomized trial of the Inter Groupe Français du Myelome (IFM) Blood 1997 90: 418a
Munshi NC, Desikan KR, Siegel D et al. Preliminary report of clinical efficacy of patient specific vaccination using purified idiotype protein in myeloma Blood 1999 94: 704a
Powles R, Mehta J, Singhal S et al. Autologous bone marrow or peripheral blood stem cell transplantation followed by maintenance chemotherapy for adult acute lymphoblastic leukemia in first remission; 50 cases from a single center Bone Marrow Transplant 1995 16: 241–247
Desikan R, Siegel D, Fassas A et al. DCEP consolidation after tandem autotransplants in high risk multiple myeloma – improved prognosis compared to matched historical controls Blood 1998 92: 660a
Acknowledgements
This work was supported in part by CA55819 from the National Cancer Institute, Bethesda, MD.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Desikan, K., Tricot, G., Dhodapkar, M. et al. Melphalan plus total body irradiation (MEL-TBI) or cyclophosphamide (MEL-CY) as a conditioning regimen with second autotransplant in responding patients with myeloma is inferior compared to historical controls receiving tandem transplants with melphalan alone. Bone Marrow Transplant 25, 483–487 (2000). https://doi.org/10.1038/sj.bmt.1702167
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702167
Keywords
This article is cited by
-
A phase I/II study of escalating doses of thalidomide in conjunction with bortezomib and high-dose melphalan as a conditioning regimen for autologous stem cell transplantation in patients with multiple myeloma
Bone Marrow Transplantation (2019)
-
Bortezomib, Dexamethasone, and High-Dose Melphalan as Conditioning for Stem Cell Transplantation in Young Japanese Multiple Myeloma Patients: A Pilot Study
Indian Journal of Hematology and Blood Transfusion (2013)
-
Phase I/II study of tandem high-dose chemotherapy with autologous peripheral blood stem cell transplantation for advanced multiple myeloma
International Journal of Hematology (2009)
-
Current status of autologous hematopoietic stem cell transplantation in myeloma
Bone Marrow Transplantation (2008)
-
High-dose chemotherapy and autologous hematopoietic stem cell transplantation in myeloma patients under the age of 65 years
Bone Marrow Transplantation (2007)