Abstract
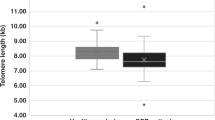
Telomere length of peripheral blood mononuclear cells (PBMCs) from 23 autologous HSCT patients ranging from 4 to 61 years old, and 46 allogeneic HSCT recipients from 6 to 52 years old were studied to confirm whether excessive shortening of telomeres is associated with HSCT. After autologous HSCT, telomere length of PBMCs ranged from 6.8 to 12.0 kb. The comparison between transplanted PBMCs and PBMCs after autologous HSCT showed shortening by up to 1.9 kb (mean ± s.d.: 0.64 ± 0.50 kb). There was a difference between autologous HSCT patients and normal volunteers in the slopes of regression lines. After allogeneic HSCT, telomere length of PBMCs ranged from 6.8 to 12.0 kb. Telomeres of recipients were up to 2.1 kb (0.60 ± 0.468 kb) shorter than those of donors. The slope of regression lines for allogeneic HSCT patients and normal volunteers were parallel. Although all patients were transplanted with more than 2.0 × 108 cells/kg, telomere length did not correlate with the number of transplanted cells. There was no significant correlation between telomere length and recovery of hematological parameters. However, three patients with an average telomere length of 6.8 kb after HSCT took a longer period to reach the normal hematological state. Taken together, these data suggest that most HSCTs are performed within the biological safety range of telomeres, while the patients who have telomeres shorter than 7.0 kb after HSCT should be observed carefully for long-term hematopoiesis and the occurrence of hematopoietic disorders. Bone Marrow Transplantation (2000) 25, 441–447.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Blackburn EH . Structure and function of telomeres Nature 1991 350: 569–573
Zaikan VA . Telomeres: beginning to understand the end Science 1995 270: 1601–1607
Harley CB, Kim NW, Prowse KR et al. Telomerase, cell immortality, and cancer Cold Spring Harb Symp Quant Biol 1994 59: 307–315
Lansdorp PM . Telomere length and proliferation potential of hematopoietic stem cells J Cell Sci 1995 108: 1–6
Harly CB . Telomere loss: mitotic clock or genetic time bomb? Mutat Res 1991 265: 271–282
Vaziri H, Schächter F, Uchida I et al. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes Am J Hum Genet 1993 52: 661–667
Vaziri H, Dragowska W, Allsopp RC et al. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age Proc Natl Acad Sci USA 1994 91: 9857–9860
Hastie ND, Dempster M, Dunlop MG et al. Telomere reduction in human colorectal carcinoma and with ageing Nature 1990 346: 866–868
Nataro R, Cimmino A, Tabanni D et al. In vivo telomere dynamics of human hematopoietic stem cells Proc Natl Acad Sci USA 1998 94: 13782–13785
Wynn RF, Cross MA, Hatton C et al. Accelerated telomere shortening in young recipients of allogeneic bone marrow transplants Lancet 1998 351: 178–181
Akiyama M, Hoshi Y, Sakurai S et al. Changes of telomere length in children after hematopoietic stem cell transplantation Bone Marrow Transplant 1998 21: 167–171
Shay JW . Accelerated telomere shortening in bone marrow recipients Lancet 1998 351: 153–154
Lee JJ, Kook H, Chung IJ et al. Telomere length in patients undergoing hematopoietic stem cell transplantation Bone Marrow Transplant 1999 24: 411–415
Frenck Jr RW, Blackburn EH, Shannon KM . The rate of telomere sequence loss in human leukocytes varies with age Proc Natl Acad Sci USA 1998 95: 5607–5610
Ball SE, Gibson FM, Rizzo S et al. Progressive telomere shortening in aplastic anemia Blood 1998 91: 3582–3592
Yamada O, Oshimi K, Motoji T, Mizoguchi H . Telomeric DNA in normal and leukemic blood cells J Clin Invest 1995 95: 1117–1123
Wynn R, Thornley I, Freedman M, Saunders EF . Telomere shortening in leukocyte subsets of long-term survivors of allogeneic bone marrow transplantation Br J Haematol 1999 105: 997–1001
Weng NP, Levine BL, June CH, Hodes RJ . Human naive and memory T lymphocytes differ in telomeric length and replicative potential Proc Natl Acad Sci USA 1995 92: 11091–11094
Kronenwett R, Murea S, Haas R . Telomere length of blood-derived mononuclear cells from cancer patients during G-CSF-enriched marrow recovery Bone Marrow Transplant 1996 18: (Suppl. 1) 10–14
Mavroudis D, Read E, Cottler-Fox M et al. CD34+ cell dose predicts survival, post-transplant morbidity, and rate of hematologic recovery after allogeneic marrow transplants for hematologic malignancies Blood 1996 88: 3223–3229
Fitzgerald PH, Morris CM . Telomeric association of chromosomes in B-cell lymphoid leukemia Hum Genet 1984 67: 385–390
Morgan R, Jarzabek V, Jaffe JP et al. Telomeric fusion in pre-T-cell acute lymphoblastic leukemia Hum Genet 1986 73: 260–263
Perot C, van den Akker J, Laporte JP et al. Multiple chromosome abnormalities in patients with acute leukemia after autologous bone marrow transplantation using total body irradiation and marrow purged with mafosfamide Leukemia 1993 10: 509–515
Testoni N, Martinelli G, Zaccaria A et al. Detection of occasional and clonal chromosome aberration in patients with acute non-lymphocytic leukemia after autologous bone marrow transplantation Bone Marrow Transplant 1996 18: 1141–1145
Giralt SA, Champlin RE . Leukemia relapse after allogeneic bone marrow transplantation: a review Blood 1994 84: 3603–3612
Elfenbein G, Brogaonkar D, Bias W et al. Cytogenetic evidence for recurrence of acute myelogenous leukemia after allogeneic bone marrow transplantation in donor hematopoietic cells Blood 1987 52: 627–636
Newburger P, Latt S, Pesando J et al. Leukemia relapse in donor cells after allogeneic bone marrow transplantation New Engl J Med 1981 304: 712–714
Acknowledgements
We thank Yoshikatsu Eto (Jikei University School of Medicine) for helpful advice and critical reading of the manuscript. This work was supported in part by the special Coordination Fund for ‘Research for the Future Program’ from the Science and Technology Agency and by a grant in aid from ‘High-Tech Research Center (Institute of DNA Medicine)’ from the Ministry of Education of Japan.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Akiyama, M., Asai, O., Kuraishi, Y. et al. Shortening of telomeres in recipients of both autologous and allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 25, 441–447 (2000). https://doi.org/10.1038/sj.bmt.1702144
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702144
Keywords
This article is cited by
-
Homeostatic proliferation leads to telomere attrition and increased PD-1 expression after autologous hematopoietic SCT for systemic sclerosis
Bone Marrow Transplantation (2018)
-
No association between donor telomere length and outcomes after allogeneic unrelated hematopoietic cell transplant in patients with acute leukemia
Bone Marrow Transplantation (2018)
-
The role of telomeres and telomerase in hematologic malignancies and hematopoietic stem cell transplantation
Journal of Hematology & Oncology (2014)
-
Comparative assessment of telomere length before and after hematopoietic SCT: role of grafted cells in determining post-transplant telomere status
Bone Marrow Transplantation (2010)
-
Telomeres, senescence, and hematopoietic stem cells
Cell and Tissue Research (2008)