Abstract
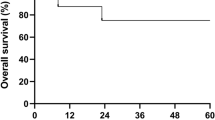
Graft-versus-host disease (GVHD) is an important complication of bone marrow transplantation after transplants between HLA-mismatched donor/recipient pairs. In mice, giving IL-2 post transplant decreases GVHD in this setting. We studied high-dose IL-2 therapy in pigs. Transplants were carried out after conditioning with fractionated total body radiation and cyclophosphamide. Fourteen pigs received a fully mismatched bone marrow transplant (six with IL-2; eight without IL-2), and six received a single haplotype class II mismatched transplant (three with IL-2; three without IL-2). GVHD was evaluated by skin histology. All fully mismatched recipients had severe GVHD (grade 2–3) and died within 13 to 51 days whether or not they received IL-2. Pigs receiving a one haplotype class II mismatched transplant without IL-2 developed severe skin GVHD lasting for 8–45 days; all died within 57 days. Similar pigs receiving IL-2 post transplant had no or only mild skin GVHD for less than 15 days; two are long-term survivors. Bone Marrow Transplantation (2000) 25, 47–52.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rodt H, Thierfelder S, Eulitz M . Anti-lymphocyte antibodies and marrow transplantation Eur J Immunol 1974 4: 15–19
Reisner Y, Kapoor N, Kirkpatrick D et al. Transplantation for acute leukemia with HLA-A and B nonidentical parental marrow cells fractionated with soybean agglutinin and sheep red blood cells Lancet 1981 2: 327–331
Filipovich AH, Vallera DA, Youle RJ et al. Ex vivo T cell depletion with immunotoxins in allogeneic bone marrow transplantation: the pilot clinical study for prevention of graft-versus-host disease Transplant Proc 1985 17: 442–444
Soderling CCB, Song CW, Blazar BR, Vallera DA . A correlation between conditioning and engraftment in recipients of MHC-mismatched T cell-depleted murine bone marrow transplants J Immunol 1985 135: 941–946
O'Reilly RJ, Collins NH, Kernan N et al. Transplantation of marrow depleted of T cells by soybean lectin agglutination and E-rosette depletion: major histocompatibility complex-related graft resistance in leukemic transplant recipients Transplant Proc 1985 17: 455–459
Martin PJ, Hansen JA, Torok-Storb B et al. Graft failure in patients receiving T cell-depleted HLA-identical allogeneic marrow transplants Bone Marrow Transplant 1988 3: 445–456
Poynton CH . T cell depletion in bone marrow transplantation Bone Marrow Transplant 1988 3: 265–279
Horovitz MM, Gale RP, Sondel PM et al. Graft-versus-leukemia reactions after bone marrow transplantation Blood 1990 75: 555–562
Anasetti C, Amos D, Beatty PG et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma New Engl J Med 1989 320: 197–204
Sykes M, Romick M, Sachs DH . Interleukin 2 prevents graft-versus-host disease while preserving the graft-versus-leukemia effect of allogeneic T cells Proc Natl Acad Sci USA 1990 87: 5633–5637
Sykes M, Romick ML, Hoyles KA, Sachs DH . In vivo administration of interleukin 2 plus T cell-depleted syngeneic marrow prevents graft-versus-host disease mortality and permits alloengraftment J Exp Med 1990 171: 645–658
Sykes M, Pearson DA . Alloengraftment in IL-2-treated mice Bone Marrow Transplant 1992 10: 157–163
Sykes M, Abraham VS, Harty MW, Pearson DA . IL-2 reduces graft-versus-host disease and preserves a graft-versus-leukemia effect by selectively inhibiting CD4+ T cell activity J Immunol 1993 150: 197–205
Sprent J, Scheafer M, Gao E, Korngold R . Role of T cell subsets in lethal graft versus host disease (GVHD) directed to class I versus class II H-2 differences. I. L3T4+ cells can either augment or retard GVHD elicited by Lyt-2+ cells in a class I-different host J Exp Med 1988 167: 556–569
Malkovski M, Brenner MK, Hunt R et al. T-cell depletion of allogeneic bone marrow prevents acceleration of graft-versus-host disease induced by exogenous interleukin-2 CellImmunol 1986 103: 476–480
Jadus MR, Peck AB . Lethal murine graft-versus-host disease in absence of detectable cytotoxic T lymphocytes Transplantation 1983 36: 281–289
Nakajima K, Smith CV, Mixon A et al. In vitro and in vivo effect of recombinant human IL-2 in miniature swine J Im-munother 1992 11: 169–175
Lunney JK, Pescovitz MD, Sachs DH . The swine major histocompatibility complex: Its structure and function. In: Tumbleson ME (ed) Swine in Biomedical Research Plenum Press: New York 1986 pp 1821–1836
Pennington LR, Sakamoto K, Popitz-Bergez FA et al. Bone marrow transplantation in miniature swine. I. Development of the model Transplantation 1988 45: 21–26
Thomas ED, Storb R, Clift RA et al. Bone-marrow transplantation New Engl J Med 1975 292: 895–902
Sykes M, Harty MW, Pearson D . Strain dependence of IL-2-induced GVHD protection: evidence that IL-2 inhibits selected CD4 function J Immunother 1994 15: 11–21
Szebeni J, Wang M-G, Pearson DA et al. IL-2 inhibits early increases in serum gamma interferon levels associated with graft-vs-host disease Transplantation 1994 58: 1385–1393
Acknowledgements
We would like to thank Drs Gary Haller and Yasushi Fuchimoto for their helpful reviews of the manuscript, and Lisa A Bernardo for help in manuscript preparation. This work was supported by a National Institutes of Health Grant No. 1 RO1 CA61537 and by a Sponsored Research Agreement between the Massachusetts General Hospital and BioTransplant, Inc. T Kozlowski is a recipient of a Sandoz Fellowship in Transplantation Award sponsored by the American Society of Transplant Physicians.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kozlowski, T., Sablinski, T., Basker, M. et al. Decreased graft-versus-host disease after haplotype mismatched bone marrow allografts in miniature swine following interleukin-2 treatment. Bone Marrow Transplant 25, 47–52 (2000). https://doi.org/10.1038/sj.bmt.1702083
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702083
Keywords
This article is cited by
-
Cytokines in Graft-Versus-Host Disease and the Graft-Versus-Leukemia Reaction
International Journal of Hematology (2001)