Abstract
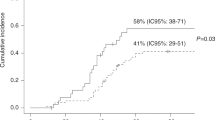
Pneumonia due to Pneumocystis carinii is an infrequent complication following autologous stem cell transplantation (ASCT) which is associated with a high mortality. Although administration of trimethoprim/sulfa- methoxazole (TMP/SMX) is an effective prophylactic strategy for Pneumocystis carinii pneumonia (PCP), treatment-associated toxicity frequently results in discontinuation of therapy. We have conducted a prospective randomized trial comparing atovaquone, a new anti-Pneumocystis agent, with TMP/SMX for PCP prophylaxis following autologous peripheral blood stem cell (PBSC) transplantation. Thirty-nine patients were studied. Twenty patients received atovaquone suspension and 19 patients received TMP/SMX. The median ages were 44 (range 20–68) and 47 (range 32–63) years, respectively. A similar number of patients with solid tumors (14 vs 15) and hematologic malignancies (five vs five) were treated in each group. Either TMP/SMX (160/800 mg) or atovaquone (1500 mg) was administered daily from transplant day −5 until day −1, discontinued from day 0 to engraftment, then resumed 3 days per week until day +100 post-transplant. The median time to engraftment (ANC >0.5 × 109/l) was similar in both groups. Eighty percent of the patients randomized to atovaquone prophylaxis completed the study. Four atovaquone-treated patients were removed from study; two patients (10%) did not receive a transplant and two patients (10%) were removed due to a protocol violation. None of the 16 patients treated with atovaquone experienced treatment-associated adverse effects. Of the 19 patients randomized to receive TMP/SMX, 55% completed the study. Nine TMP/SMX-treated patients were removed from the study; one patient (5%) did not receive a transplant and eight patients (40%) were removed due to drug intolerance (P < 0.003). the rate of intolerance to tmp/smx led to the early discontinuation of this randomized trial. intolerance of tmp/smx included elevated transaminase levels (n = 1), nausea or vomiting (n = 3), thrombocytopenia (n = 2) and neutropenia (n = 2). All episodes of TMP/SMP intolerance occurred following transplantation after a median duration of 17.5 (range 2–48) days and a median of 7 (range 1–20) doses. Resolution of adverse side-effects occurred in all eight patients within a median of 7 (range 2–20) days following discontinuation of therapy. Neither PCP nor bacterial infections were identified in any of the patients treated. This prospective randomized study demonstrated that atovaquone is well-tolerated for anti-Pneumocystis prophylaxis in autologous PBSC transplant patients intolerant of TMP/SMX.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Colby, C., McAfee, S., Sackstein, R. et al. A prospective randomized trial comparing the toxicity and safety of atovaquone with trimethoprim/sulfamethoxazole as Pneumocystis carinii pneumonia prophylaxis following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 24, 897–902 (1999). https://doi.org/10.1038/sj.bmt.1702004
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702004
Keywords
This article is cited by
-
Feasibility of trimethoprim/sulfamethoxazole desensitization therapy in hematological diseases
Clinical and Experimental Medicine (2022)
-
Comparative effectiveness of trimethoprim-sulfamethoxazole versus atovaquone for the prophylaxis of pneumocystis pneumonia in patients with connective tissue diseases receiving prolonged high-dose glucocorticoids
Rheumatology International (2022)
-
Primary prophylaxis of bacterial infections and Pneumocystis jirovecii pneumonia in patients with hematologic malignancies and solid tumors: 2020 updated guidelines of the Infectious Diseases Working Party of the German Society of Hematology and Medical Oncology (AGIHO/DGHO)
Annals of Hematology (2021)
-
Radiation pneumonitis complicated by Pneumocystis carinii in patients with thoracic neoplasia: a clinical analysis of 7 cases
Cancer Communications (2019)
-
Management of infection and febrile neutropenia in patients with solid cancer
Clinical and Translational Oncology (2016)