Abstract
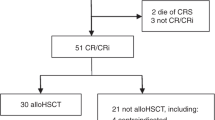
Breast cancer patients with more than three involved axillary lymph have a high likelihood of relapse after adjuvant therapy. Early results of administration of high-dose chemotherapy (HDCT) and autologous peripheral blood progenitor cells (PBPC) to patients with primary breast cancer and ⩾10 involved axillary nodes have been encouraging. We performed a multicenter trial to determine whether HDCT could be safely administered to patients with primary breast cancer involving 4–9 axillary lymph nodes. Fifty-four patients with stage II or III breast cancer and 4–9 involved axillary lymph nodes received doxorubicin-based induction chemotherapy, followed by high-dose cyclophosphamide (5.625 g/m2), cisplatin (165 mg/m2), and BCNU (450 mg/m2) and PBPC mobilized by sargramostim (GM-CSF) or filgrastim (G-CSF). After completion of HDCT, patients received radiation therapy to the chest wall or involved breast, plus tamoxifen. Survival and disease-free survival, time to engraftment, and charges associated with HDCT were determined. Plasma concentrations of BCNU were determined and plasma AUCBCNU was calculated. Fifty-four patients were evaluable for survival and relapse-free survival. Fifty-two patients received HDCT with PBPC support and were evaluable for toxicity. Fifteen patients (29%) developed late pulmonary drug toxicity, which resolved with a 10-week course of corticosteroids in all but one affected patient, who subsequently died of pulmonary toxicity. Ten patients relapsed a median of 426 days (range 86–1117 days) after the start of induction chemotherapy, seven of whom have died. Forty-three patients are alive and breast cancer-free at a median of 947 days (range 661–1730 days) after the start of therapy, including one patient who developed myelodysplastic syndrome 809 days after the start of HDCT. Actuarial 4-year survival and disease-free survival from the start of treatment are 84 and 71%, respectively. Mean plasma AUCBCNU was 400 (range 82–1255) μ g.min/ml and was not statistically different from that measured in historical controls who received 600 mg/m2 of BCNU. Combined hospital and physician charges for patients treated at the University of Colorado decreased from a mean of $125 845 for the first four patients to $77 126 for the final seven patients. We conclude that HDCT with autologous PBPC can be administered with acceptable safety to patients with primary breast cancer involving 4–9 axillary lymph nodes. An ongoing, prospective randomized trial is evaluating the efficacy of HDCT for this patient group.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bearman, S., Overmoyer, B., Bolwell, B. et al. High-dose chemotherapy with autologous peripheral blood progenitor cell support for primary breast cancer in patients with 4–9 involved axillary lymph nodes. Bone Marrow Transplant 20, 931–937 (1997). https://doi.org/10.1038/sj.bmt.1701000
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1701000
Keywords
This article is cited by
-
Carmustine infusion reactions are more common with rapid administration
Supportive Care in Cancer (2012)
-
Prognostic analysis of tumour angiogenesis, determined by microvessel density and expression of vascular endothelial growth factor, in high-risk primary breast cancer patients treated with high-dose chemotherapy
British Journal of Cancer (2007)
-
Stem-cell transplantation for the treatment of advanced solid tumors
Springer Seminars in Immunopathology (2004)
-
Adjuvant treatment of high-risk stage II breast cancer with doxorubicin followed by high-dose chemotherapy and autologous stem-cell transplantation: a single-institution experience with 132 consecutive patients
Bone Marrow Transplantation (2003)
-
Interferon-γ for delayed pulmonary toxicity syndrome resistant to steroids
Bone Marrow Transplantation (2003)