Abstract
Objective:
To study the effect of sea buckthorn berries on the number and duration of common cold (CC) infections. As secondary objectives the effects on digestive and urinary tract infections (DTI, UTI), and serum C-reactive protein (CRP) concentrations were also investigated.
Subjects:
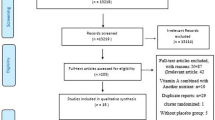
A total of 254 healthy volunteers were randomly assigned to receive sea buckthorn or placebo product during the study, which 233 of them completed.
Results:
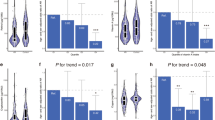
There were no significant differences in the number or duration of CC or DTI between groups (CC: relative risks (sea buckthorn vs placebo) for the number and duration were 1.15 (95% CI 0.90–1.48) and 1.05 (95% CI 0.87–1.27), respectively). In the sea buckthorn group, as compared to the placebo, the serum CRP concentrations decreased significantly (difference in median change −0.059 mg/l, P=0.039). The number of UTI was too small to draw solid conclusions, but the results indicate the subject merits further investigation.
Conclusion:
Sea buckthorn berries did not prevent CC or DTI. However, a reductive effect on CRP, a marker of inflammation, and a risk factor for cardiovascular diseases, was detected.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ågren JJ, Julkunen A, Penttilä I (1992). Rapid separation of serum lipids for fatty acid analysis by a single aminopropyl column. J Lipid Res 33, 1871–1876.
Barrett BP, Brown RL, Locken K, Maberry R, Bobula JA, D’Alessio D (2002). Treatment of the common cold with unrefined echinacea. Ann Intern Med 137, 939–946.
Brinkworth GD, Buckley JD (2003). Concentrated bovine colostrum protein supplementation reduces the incidence of self-reported symptoms of upper respiratory tract infection in adult males. Eur J Nutr 42, 228–232.
Caruso TJ, Gwaltney JM (2005). Treatment of the common cold with echinacea: a structured review. Clin Infect Dis 40, 807–810.
Conti C, Mastromarino P, Sgro R, Desideri N (1998). Anti-picornavirus activity of synthetic flavon-3-yl esters. Antivir Chem Chemother 9, 511–515.
de Ancos B, Gonzalez EM, Cano MP (2000). Ellagic acid, vitamin C, and total phenolic contents and radical scavenging capacity affected by freezing and frozen storage in raspberry fruit. J Agric Food Chem 48, 4565–4570.
Desbiens NA (2002). In randomized controlled trials, should subjects in both placebo and drug groups be expected to guess that they are taking drug 50% of the time? Med Hypotheses 59, 227–232.
Dorhoi A, Dobrean V, Zahan M, Virag P (2006). Modulatory effects of several herbal extracts on avian peripheral blood cell immune responses. Phytother Res 20, 352–358.
Eccleston C, Baoru Y, Tahvonen R, Kallio H, Rimbach GH, Minihane AM (2002). Effects of an antioxidant-rich juice (sea buckthorn) on risk factors for coronary heart disease in humans. J Nutr Biochem 13, 346–354.
Finnish National Nutrition Council (2005). Suomalaiset ravitsemussuositukset – ravinto ja liikunta tasapainoon, 2005. Edita Publishing: Helsinki, Finland. Available on: http://www.b.mmm.fi/ravitsemusneuvottelukunta/Suositus98_lyh.htm.
Gao X, Ohlander M, Jeppsson N, Bjork L, Trajkovski V (2000). Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J Agric Food Chem 48, 1485–1490.
Geetha S, Sai Ram M, Singh V, Ilavazhagan G, Sawhney RC (2002). Anti-oxidant and immunomodulatory properties of seabuckthorn (Hippophae rhamnoides) – an in vitro study. J Ethnopharmacol 79, 373–378.
Gwaltney JM, Buier RM, Rogers JL (1996). The influence of signal variation, bias, noise, and effect size on statistical significance in treatment studies of the common cold. Antiviral Res 29, 287–295.
Häkkinen SH, Kärenlampi SO, Mykkänen HM, Törrönen AR (2000). Influence of domestic processing and storage on flavonol contents in berries. J Agric Food Chem 48, 2960–2965.
Heikkinen T, Järvinen A (2003). The common cold. Lancet 361, 51–59.
Hemilä H, Chalker E, D’Souza R, Douglas R, Treacy B (2004). Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 4, CD000980.
Hemilä H, Kaprio J, Albanes D, Heinonen OP, Virtamo J (2002). Vitamin C, vitamin E, and beta-carotene in relation to common cold incidence in male smokers. Epidemiology 13, 32–37.
Johansson AK, Korte H, Yang BR, Stanley JC, Kallio HP (2000). Sea buckthorn berry oil inhibits platelet aggregation. J Nutr Biochem 11, 491–495.
Kallio H, Yang B, Halttunen T (2005). Flavonol glycosides of berries of three major sea buckthorn subspecies, Hippophaë rhamnoides ssp. rhamnoides, ssp. sinensis and ssp. Mongolica. The proceedings of invited speeches of the Second International Seabuckthorn Association Conference; 26–29 August 2005; Beijing, China.
Kallio H, Yang B, Peippo P (2002a). Effects of different origins and harvesting time on vitamin C, tocopherols, and tocotrienols in sea buckthorn (Hippophaë rhamnoides) berries. J Agric Food Chem 50, 6136–6142.
Kallio H, Yang BR, Peippo P, Tahvonen R, Pan RL (2002b). Triacylglycerols, glycerophospholipids, tocopherols, and tocotrienols in berries and seeds of two subspecies (ssp sinensis and mongolica) of sea buckthorn (Hippophaë rhamnoides). J Agric Food Chem 50, 3004–3009.
Kontiokari T, Laitinen J, Järvi L, Pokka T, Sundqvist K, Uhari M (2003). Dietary factors protecting women from urinary tract infection. Am J Clin Nutr 77, 600–604.
Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Koskela M, Uhari M (2001). Randomised trial of cranberry-lingonberry juice and lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ 322, 1571.
Langkamp-Henken B, Bender BS, Gardner EM, Herrlinger-Garcia KA, Kelley MJ, Murasko DM et al. (2004). Nutritional formula enhanced immune function and reduced days of symptoms of upper respiratory tract infection in seniors. J Am Geriatr Soc 52, 3–12.
Manach C, Scalbert A, Morand C, Remesy C, Jimenez L (2004). Polyphenols: food sources and bioavailability. Am J Clin Nutr 79, 727–747.
Männistö S, Ovaskainen ML, Valsta L (2003). Finnravinto 2002 – tutkimus. The National FINDIET 2002 Study. Publications of the National Public Health Institute B3/2003. The National Public Health Institute, Nutrition Unit. Helsinki, Finland. Available on: http://www.ktl.fi/portal/suomi/osastot/eteo/yksikot/ravitsemusyksikko/finravinto_-tutkimus/finravinto_2002_-tutkimuksen_raportti/.
Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT, Singh MF et al. (2004). Vitamin E and respiratory tract infections in elderly nursing home residents – a randomized controlled trial. JAMA 292, 828–836.
Middleton EJ, Kandaswami C, Theoharides T (2000). The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52, 673–751.
Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM et al. (2006). Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 83, 1369–1379.
Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA (2001). Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 74, 418–425.
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon III RO, Criqui M et al. (2003). Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107, 499–511.
Pepys MB, Hirschfield GM (2003). C-reactive protein: a critical update. J Clin Invest 111, 1805–1812.
Puhakka T, Pitkäranta A, Ruuskanen O (2000). Flunssa ja sen komplikaatiot. Duodecim 116, 39–45.
Puupponen-Pimiä R, Nohynek L, Alakomi HL, Oksman-Caldentey KM (2005). Bioactive berry compounds-novel tools against human pathogens. Appl Microbiol Biotechnol 67, 8–18.
Puupponen-Pimiä R, Nohynek L, Meier C, Kähkönen M, Heinonen M, Hopia A et al. (2001). Antimicrobial properties of phenolic compounds from berries. J Appl Microbiol 90, 494–507.
Rees JR, Wade TJ, Levy DA, Colford Jr JM, Hilton JF (2005). Changes in beliefs identify unblinding in randomized controlled trials: a method to meet CONSORT guidelines. Contemp Clin Trials 26, 25–37.
Rein D, Schijlen E, Kooistra T, Herbers K, Verschuren L, Hall R et al. (2006). Transgenic flavonoid tomato intake reduces C-reactive protein in human C-reactive protein transgenic mice more than wild-type tomato. J Nutr 136, 2331–2337.
Tiitinen K, Hakala M, Kallio H (2005). Quality components of sea buckthorn (Hippophaë rhamnoides) varieties. J Agric Food Chem 53, 1692–1699.
Tiitinen K, Vahvaselkä M, Hakala M, Laakso S, Kallio H (2006). Malolactic fermentation in sea buckthorn (Hippophaë rhamnoides L.) juice processing. Eur Food Res Techol 222, 686–691.
Turner RB, Fowler SL, Berg K (2004). Treatment of the common cold with troxerutin. APMIS 112, 605–611.
van Oostrom AJ, van Wijk J, Cabezas MC (2004). Lipaemia, inflammation and atherosclerosis: novel opportunities in the understanding and treatment of atherosclerosis. Drugs 64 (Suppl 2), S19–S41.
Wannamethee SG, Lowe GD, Rumley A, Bruckdorfer KR, Whincup PH (2006). Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am J Clin Nutr 83, 567–574.
Yang BR, Kallio H (2002). Composition and physiological effects of sea buckthorn (Hippophaë) lipids. Trends Food Sci Technol 13, 160–167.
Yang BR, Kalimo KO, Mattila LM, Kallio SE, Katajisto JK, Peltola OJ et al. (1999). Effects of dietary supplementation with sea buckthorn (Hippophaë rhamnoides) seed and pulp oils on atopic dermatitis. J Nutr Biochem 10, 622–630.
Acknowledgements
We thank the volunteers who participated in the study. We also thank Terhi Pohjanheimo and Katja Tiitinen for development of the treatment products; Nina Kainulainen, Raija Nurmi, and Anja Pirinen for excellent assistance; Jukka-Pekka Suomela for the flavonol glycoside analysis of the study product; Saija Hurme for the statistical analyses of CRP data; and Marjo Mäkinen-Aakula and Ilkka Liukas for their contribution. We acknowledge the Finnish Agency for Technology and Innovation, Pakkasmarja Inc., Riitan Herkku Inc., Valioravinto Inc., Vinkkilä Organic Product, Turku University Foundation and ABS Graduate School for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guarantor: R Tahvonen.
Contributors: RT was responsible for the study concept, supervision and design. PL contributed to study design and was responsible for recruitment of the participants, data collection and entry, writing the manuscript and with RT and JA for analysis of data and interpretation of results. JA was responsible for the sample size estimation, randomization of the participants, statistical analysis of the data, and contributed to writing the paper. ES and HK contributed to the design of the study. RT, ES and HK revised the manuscript critically for important intellectual content.
Supplementary Information accompanies the paper on European Journal of Clinical Nutrition website (http://www.nature.com/ejcn)
Supplementary information
Rights and permissions
About this article
Cite this article
Larmo, P., Alin, J., Salminen, E. et al. Effects of sea buckthorn berries on infections and inflammation: a double-blind, randomized, placebo-controlled trial. Eur J Clin Nutr 62, 1123–1130 (2008). https://doi.org/10.1038/sj.ejcn.1602831
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602831
Keywords
This article is cited by
-
Fatty acids in berry lipids of six sea buckthorn (Hippophae rhamnoides L., subspecies carpatica) cultivars grown in Romania
Chemistry Central Journal (2012)
-
Different berries and berry fractions have various but slightly positive effects on the associated variables of metabolic diseases on overweight and obese women
European Journal of Clinical Nutrition (2011)
-
Berry meals and risk factors associated with metabolic syndrome
European Journal of Clinical Nutrition (2010)
-
Postprandial hyperglycemia and insulin response are affected by sea buckthorn (Hippophaë rhamnoides ssp. turkestanica) berry and its ethanol-soluble metabolites
European Journal of Clinical Nutrition (2010)
-
Effect of a low dose of sea buckthorn berries on circulating concentrations of cholesterol, triacylglycerols, and flavonols in healthy adults
European Journal of Nutrition (2009)