Abstract
Objective:
To investigate the long-term effect of oral magnesium supplementation on clinical symptoms, bronchial reactivity, lung function and allergen-induced skin responses in children and adolescents with moderate persistent asthma.
Design:
A double-blind randomized parallel placebo-controlled study.
Setting and subjects:
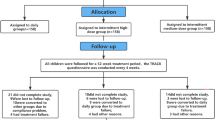
The patients were recruited from the Pediatric Outpatient Clinic, Division of Pulmonology, Allergy and Immunology, and followed at the Center for Investigation in Pediatrics at State University of Campinas Hospital, Brazil. Thirty-seven out of 72 patients met the study criteria. There were no dropouts.
Intervention:
The 37 patients (aged 7–19 years, 19 males) were randomized in two groups: magnesium (n=18, 300 mg/day) and placebo (n=19), during 2 months. Both patient groups received inhaled fluticasone (250 μg twice a day) and salbutamol as needed. The primary outcome was bronchial reactivity evaluated with methacholine challenge test (PC20).
Results:
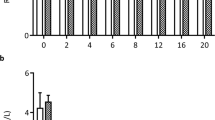
After a follow-up of 2 months, the methacholine PC20 for testing bronchial reactivity has augmented significantly in the magnesium group only. The skin responses to recognized antigens have also decreased in patients treated with magnesium. The forced vital capacity (FVC), the forced expiratory volume at first second (FEV1), the forced expiratory flow at 25–75 and the FEV1/FVC ratio were similar in both groups. The magnesium group presented fewer asthma exacerbations and used less salbutamol compared to the placebo group.
Conclusions:
Oral magnesium supplementation helped to reduce bronchial reactivity to methacholine, to diminish their allergen-induced skin responses and to provide better symptom control in pediatric patients with moderate persistent asthma treated with inhaled fluticasone.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Altura BM, Altura BT (1991–92). Cardiovascular risk factors and magnesium relationships to atherosclerosis, ischemic heart disease and hypertension. Magnesium Trace Elem 10, 182–192.
Altura BM, Altura BT, Gebrewold A, Ising H, Gunther T (1984). Magnesium deficiency and hypertension: correlation between magnesium-deficient diets and microcirculatory changes in situ. Science 223, 1315–1317.
Bois P (1963). Effect of magnesium deficiency on mast cells and urinary histamine in rats. Br J Exp Pathol 44, 151–155.
Britton J, Pavord I, Richards K, Wisniewski A, Knox A, Lewis S et al. (1994). Dietary magnesium, lung function, wheezing and airway hyperreactivity in a random adult population sample. Lancet 344, 357–362.
Chadwick DJ, Cardew C (eds) (1997). The Rising Trends in Asthma, CIBA Foundation Symposium No. 206. Wiley: New York.
Ciarallo L, Sauer AH, Shannon MW (1996). Intravenous magnesium therapy for moderate to severe pediatric asthma: results of a randomized, placebo-controlled trial. J Pediatr 129, 809–814.
Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG et al. (2000). American Thoracic Society: guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 161, 309–329.
Del Castillo J, Engbaek L (1954). The nature of neuromuscular block produced by magnesium. J Physiol 124, 370–384.
Demoly P, Michel FB, Bousquet J (1998). In vivo methods for study of allergy skin tests, techniques, and interpretation In: Middleton E et al (eds). Allergy, Principles and Practice 5th edn.,Chapter 32, Mosby: St Louis, MO, USA,. pp 430–439.
Fantidis P, Cacho JR, Marin M, Jarabo RM, Solera J, Herrero E (1995). Intracellular (polymorphonuclear) magnesium content in patients with bronchial asthma between attacks. J R Soc Med 88, 441–445.
Fogarty A, Lewis SA, Scrivener SL, Antoniak M, Pacey S, Pringle M et al. (2003). Oral magnesium and vitamin C supplements in asthma: a parallel group randomized placebo-controlled trial. Clin Exp Allergy 33, 1355–1359.
Hill J, Micklewright A, Lewis S, Britton J (1997). Investigation of the effect of short-term change in dietary magnesium intake in asthma. Eur Respir J 10, 2225–2229.
Kemp PA, Gardiner SM, March JE, Bennett T, Rubin PC (1994). Effects of NG-nitro-L-arginine methyl ester on regional haemodynamic responses to MgSO4 in conscious rats. Br J Pharmacol 111, 325–331.
Milliken GA, Johnson DE 1984. Analysis of Messy Data. Volume I: Designed Experiments. Van Nostrand Reinhold Company: New York.
Montgomery DC (1991). Design and Analysis of Experiments, 3rd edn. Wiley: New York.
Nadler JL, Goodson S, Rude RK (1987). Evidence that prostacyclin mediates the vascular action of magnesium in humans. Hypertension 9, 379–383.
National Institutes of Health (2002). Global Strategy for Asthma Management and Prevention. National Heart, Lung, and Blood Institute: Bethesda, MD, USA. Guidelines for the diagnosis and manegement of asthma.www.ginasthma.com.
National Research Council (US) (1989). Recommended Dietary Allowances, 10th edn. National Academy Press: Washington, DC.
Noppen M, Vanmaele L, Impens N, Schandevyl W (1990). Bronchodilating effect of intravenous magnesium sulfate in acute severe bronchial asthma. Chest 97, 373–376.
Peto R, Pike MC, Armitage P (1976). Design and analysis of randomized clinical trials requiring prolonged observation of each patient. Br J Cancer 34, 585–612.
Plaisance P, Hibon A, Adnet F, Bouxiere D, Richard C, Payen B (1994). Potentiation of beta2-agonists by inhaled magnesium sulfate in prehospital management of acute bronchial asthma: a double-blind study. Am J Respir Crit Care Med 149, A190 (abstract).
Polgar C, Promadhat V (1971). Pulmonary Function Testing in Children: Techniques and Standards. WB Saunders: Philadelphia.
Quame GA (1993). Laboratory evaluation of magnesium status. Renal function and free intracellular magnesium concentration. Clin Lab Med 13l, 209–223.
Rosello HJ, Pla JC (1936). Sulfato de magnesio en la crisis de asma. Prensa Med Argent 23, 1677–1680.
Saris NEL, Mervaala E, Karppanen H, Khawaja JÁ, Lewenstam A (2000). Magnesium: an update on physiological, clinical and analytical aspects. Clin Chim Acta 294, 1–26.
Silverman RA, Osborn H, Runge J, Gallagher EJ, Chiang W, Feldman J et al. (2002). IV magnesium sulfate in the treatment of acute severe asthma: a multicenter randomized controlled trial. Chest 122, 489–497.
Soutar A, Seaton A, Brown K (1997). Bronchial reactivity and dietary antioxidants. Thorax 52, 166–170.
Spivey WH, Skobeloff EM, Levin RM (1990). Effect of magnesium chloride on rubbit bronchial smooth muscle. Ann Emerg Med 19, 1107–1112.
Tam M, Gomez S, Gonzalez-Gross M, Marcos A (2003). Possible roles of magnesium on the immune system, [review]. Eur J Clin Nutr 57, 1193–1197.
Trendelenburg F (1912). Physiologische and Pharmakologische untersuchungen an der Isolierten Bronchialmuskulatur. Arch Exp Pharmacol Ther 69, 79–107.
USDA (1990). Continuing Survey of Food Intake by Individuals 1989 and 1990. USDA Public Use Data Tape USDA: Washington, DC.
Zervas E, Loukides S, Papatheodorou G, Psathakis K, Tsindiris P, Panagou P et al. (2000). Magnesium levels in plasma and erythrocytes before and after histamine challenge. Eur Respir J 16, 621–625.
Acknowledgements
We thank the statistical advice of Helymar Machado and Cleide M Silva, Albion Laboratory for providing magnesium, GlaxoSmitKline for providing fluticasone and salbutamol and Ao-Pharmacêutico for drug manipulation. C Gontijo-Amaral was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES. Ministry of Education, Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guarantors: C Gontijo-Amaral and JD Ribeiro.
Contributors: CG-A conceived the idea and was responsible for study design, execution, data analysis and preparation of the paper. JDR coordinated and supervised the clinical trial. AC-N assisted with preparation of the paper and data analysis. LSCG assisted with preparation of the paper. MAGOR helped to perform pulmonary function tests.
Rights and permissions
About this article
Cite this article
Gontijo-Amaral, C., Ribeiro, M., Gontijo, L. et al. Oral magnesium supplementation in asthmatic children: a double-blind randomized placebo-controlled trial. Eur J Clin Nutr 61, 54–60 (2007). https://doi.org/10.1038/sj.ejcn.1602475
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602475
Keywords
This article is cited by
-
The role of oral magnesium supplements for the management of stable bronchial asthma: a systematic review and meta-analysis
npj Primary Care Respiratory Medicine (2019)
-
The role of magnesium in different inflammatory diseases
Inflammopharmacology (2019)
-
Serum Total Magnesium Level and its Correlation with Symptom Control in Children with Mild Persistent Asthma
The Indian Journal of Pediatrics (2018)
-
Searching for Better Asthma Control?
The Indian Journal of Pediatrics (2018)
-
Clinical pharmacokinetics of magnesium sulfate in the treatment of children with severe acute asthma
European Journal of Clinical Pharmacology (2017)