Abstract
Objective:
To examine the effect of nucleotide (NT)-supplemented cow's milk-based formula on growth and biochemical indices of immune function in healthy infants.
Design:
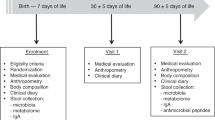
Randomized controlled trial (RCT) of formula-fed term infants allocated to control formula with an innate level of NT at 10 mg/l (n=102), or formula fortified with NT at 33.5 mg/l (n=98). A parallel group of 125 breastfed infants followed the same protocol as a reference.
Outcome measures:
Growth was assessed at enrolment, 7 weeks, 4 months and 7 months of age. Natural killer cell activity, cytokine production and lymphocyte subpopulations were assessed at 7 weeks of age. Antibody responses to diphtheria toxoid, tetanus toxoid and Haemophilus influenzae type b (Hib) immunizations were measured at 7 months of age.
Results:
NT supplementation did not influence the growth of formula fed infants or any markers of immunity measured at 7 weeks of age. Antibody responses to tetanus toxoid were higher in the NT-supplemented group (n=68) compared with the control group (n=70) at 7 months of age (median (5th, 95% percentile): 1.57(0.42, 3.43) vs 1.01(0.41, 4.66) IU/ml, P<0.03). A difference between treatments was seen in response to diphtheria toxoid but this effect disappeared when adjusted for hepatitis B immunization at birth. There was no effect of treatment on antibody responses to Hib immunization.
Conclusions:
Supplementation of formulas with NT at 33.5 mg/l resulted in a modest improvement in antibody response consistent with RCTs that used higher levels of NT supplementation. Whether this translates to clinical benefits in well-nourished infants requires further study.
Sponsorship:
Supported by a grant from Wyeth Nutrition. Dr Makrides was supported by an RD Wright Fellowship from the National Health and Medical Research Council of Australia and Dr Gibson was partially supported by the MS McLeod Research Trust and a Senior Research Fellowship from the National Health and Medical Research Council of Australia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Aggett P, Leach JL, Rueda R, MacLean Jr WC (2003). Innovation in infant formula development: a reassessment of ribonucleotides in 2002. Nutrition 19, 375–384.
Australian Technical Advisory Group on Immunisation NH&MRC (2000). The Australian Immunisation Handbook, 7th edn. Australian Government Publishing Service.
Balmer SA, Hanvey LS, Wharton BA (1994). Diet and faecal flora in the newborn: nucleotides. Arch Dis Child 70, F137.
Brunser O, Espinoza J, Araya M, Cruchet S, Gil A (1994). Effect of dietary nucleotide supplementation on diarrhoeal disease in infants. Acta Paediatr 83, 188–191.
Buck RH, Thomas DL, Winship TR, Cordle CT, Kuchan MJ, Baggs GE et al. (2004). Effect of dietary ribonucleotides on infant immune status. Part 2: immune cell development. Pediatr Res 56, 891–900.
Carver JD (1999). Dietary nucleotides: effects on the immune and gastrointestinal systems. Acta Paediatr Suppl 430, 83–88.
Carver JD, Pimentel B, Cox WI, Barness LA (1991). Dietary nucleotide effects upon immune function in infants. Pediatrics 88, 359–363.
Carver JD, Walker WA (1995). The role of nucleotides in human nutrition. Nutr Biochem 6, 58–72.
Chang L, Gusewitch GA, Chritton DB, Folz JC, Lebeck LK, Nehlsen-Cannarella SL (1993). Rapid flow cytometric assay for the assessment of natural killer cell activity. J Immunol Methods 166, 45–54.
Comans-Bitter WM, De Groot R, Van den Beemd R, Neijens HJ, Hop WCJ, Groeneveld K et al. (1997). Immunophenotyping of blood lymphocytes in childhood – reference values for lymphocyte subpopulations. J Pediatr 130, 388–393.
Cordle CT, Winship TR, Schaller JP, Thomas DJ, Buck RH, Ostrom KM et al. (2002). Immune status of infants fed soy-based formulas with or without added nucleotides for 1 year: Part 2: immune cell populations. J Pediatr Gastroenterol Nutr 34, 145–153.
Cosgrove M, Davies DP, Jenkins HR (1996). Nucleotide supplementation and the growth of term small for gestational age infants. Arch Dis Child 74, F122.
Daniel A (1983). Power, Privilege and Prestige: Occupations in Australia, 1st edn. Longman-Cheshire: Melbourne.
Decker MD, Edwards KM, Bradley R, Palmer P (2000). Comparative trial in infants of four conjugate Haemophilus influenzae type b vaccines. J Pediatr 120, 184–189.
DeLucchi C, Pita ML, Faus MJ, Molina JA, Uauy R, Gil A (1987). Effects of dietary nucleotides on the fatty acid composition of erythrocyte membrane lipids in term infants. J Pediatr Gastroenterol Nutr 6, 568–574.
Erkeller-Yuksel FM, Deneys V, Yuksel B, Hannet I, Hulstaert F, Hamilton C et al. (1992). Age-related changes in human blood lymphocyte subpopulations. J Pediatr 120, 216–222.
Food Standards Australia and New Zealand (2004). Standard 2.9.1. Infant Formula Products. Australia New Zealand Food Standards Code, Incorporating Amendmendments up to and Including Amendment 70. Anstat Pty Ltd: Melbourne.
Galazka AM (1993). The Immunological Basis for Immunization Series. Global Programme for Vaccines and Immunization. Expanded Programme on Immunization. World Health Organization: Geneva.
Gil A (2002). Modulation of the immune response mediated by dietary nucleotides. Eur J Clin Nutr 56 (Suppl 3), S1–S4.
Gil A, Corral E, Martinez A, Molina JA (1986). Effects of the addition of nucleotides to an adapted milk formula on the microbial pattern of faeces in at term newborn infants. J Clin Nutr Gastroenterol 1, 127–132.
Hawkes JS, Bryan D-L, James MJ, Gibson RA (1999b). Cytokines (IL-1β, IL-6, TNFα, TGFβ1 and TGFβ2) and prostaglandin E2 in human milk during the first three months postpartum. Pediatr Res 46, 194–199.
Hawkes JS, Neumann MA, Gibson RA (1999a). The effect of breast feeding on lymphocyte subpopulations in healthy term infants at 6 months of age. Pediatr Res 45, 648–651.
Heldrup J, Kalm O, Prellner K (1992). Blood T and B lymphocyte subpopulations in healthy infants and children. Acta Paediatr 81, 125–132.
Insel R, Anderson P (1976). Haemophilus influenzae type b: assays for the capsular polysaccharide and for anti-polysaccharide antibodies. In: Rose NR (ed). Manual of Clinical Immunology, 3rd edn. American Society for Microbiology: Washington, DC, pp 379–384.
Leach JL, Baxter JH, Molitor BE, Ramstack MB, Masor ML (1995). Total potentially available nucleosides of human milk by stage of lactation. Am J Clin Nutr 61, 1224–1230.
Martinez-Augustin O, Boza JJ, Navarro J, Martinez-Valverde A, Araya M, Gil A (1997). Dietary nucleotides may influence the humoral immunity in immunocompromised children. Nutrition 13, 465–469.
McVernon J, Andrews N, Slack M, Ramsay M (2003). Risk of vaccine failure after Haemophilus influenzae type b (Hib) combination vaccines with acellular pertussis. Lancet 361, 1521–1523.
Melville-Smith ME, Seagroatt VA, Watkins JT (1983). A comparison of enzyme-linked immunosorbent assay (ELISA) with the toxin neutralization test in mice as a method for the estimation of tetanus antitoxin in human sera. J Biol Stand 11, 137–144.
Navarro J, Maldonado J, Narbona E, Ruiz-Bravo A, Garcia Salmeron JL, Molina JA et al. (1999). Influence of dietary nucleotides on plasma immunoglobulin levels and lymphocyte subsets of preterm infants. Biofactors 10, 67–76.
Ostrom KM, Cordle CT, Schaller JP, Winship TR, Thomas DJ, Jacobs JR et al. (2002). Immune status of infants fed soy-based formulas with or without added nucleotides for 1 year: Part 1: vaccine responses, and morbidity. J Pediatr Gastroenterol Nutr 34, 137–144.
Pickering LK, Granoff DM, Erickson JR, Masor ML, Cordle CT, Schaller JP et al. (1998). Modulation of the immune system by human milk and infant formula containing nucleotides. Pediatrics 101, 242–249.
Raiten DJ, Talbot JM, Waters JH (1998). Assessment of nutrient requirements for infant formulas. J Nutr 128, S2110–S2130.
Schaller JP, Kuchan MJ, Thomas DL, Cordle CT, Winship TR, Buck RH et al. (2004). Effect of dietary ribonucleotides on infant immune status. Part 1: humoral responses. Pediatr Res 56, 883–890.
Walory J, Grzesiowski P, Hryniewicz W (2000). Comparison of four serological methods for the detection of diphtheria anti-toxin antibody. J Immunol Methods 245, 55–65.
Woltil HA, van Beusekom CM, Siemensma AD, Muskiet FAJ, Okken A (1995). Dietary ribonucleotides and neonatal LCPUFA status. Am J Clin Nutr 62, 943–949.
Yau KI, Huang CB, Chen W, Chen SJ, Chou YH, Huang FY et al. (2003). Effect of nucleotides on diarrhea and immune responses in healthy term infants in Taiwan. J Pediatr Gastroenterol Nutr 36, 37–43.
Acknowledgements
We thank Suzanne McCusker, Jenni Scambiatterra, Heather Garreffa, Jo White, Jo Collins, Judy Bettes, Kathy Byrt, Sarah Russell, Elizabeth Roberton, Warren Higgs, Michelle Clarke, Lisa Smithers, Jacki Aldis, Helen Marshall, Ela Zielinski, Mark Neumann, Julie Lewis and the staff of the postnatal ward at the WCH and Lindy Harkness and the staff of the immunization clinic at the WCH for their administrative, clinical and technical support. Financial support and formula for the study came from Wyeth Nutrition and William Goldman, Kathryn Pramuk and Paul Zimmer made contributions to the study design and provided valuable comments on the manuscript. Source document verification was made by a representative from Wyeth Nutrition.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guarantors: JS Hawkes, RA Gibson and M Makrides.
Contributors: JSH, RAG and MM designed the study and were the steering committee who monitored and managed the trial. MM chaired the steering committee and managed the clinical aspects of the trial. JSH managed the laboratory analyses and wrote the report with contributions from all co-authors. DR was responsible for the immunization response analyses. All data were managed, analysed and interpreted by the trial steering committee in Adelaide.
Rights and permissions
About this article
Cite this article
Hawkes, J., Gibson, R., Roberton, D. et al. Effect of dietary nucleotide supplementation on growth and immune function in term infants: a randomized controlled trial. Eur J Clin Nutr 60, 254–264 (2006). https://doi.org/10.1038/sj.ejcn.1602310
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602310
Keywords
This article is cited by
-
Schizophyllum commune-derived β-glucan improves intestinal health demonstrating protective effects against constipation and common metabolic disorders
Applied Biological Chemistry (2022)
-
Clinical benefits of β-glucan supplementation in children: a review
Discover Food (2022)
-
Effects of dietary nucleotide supplementation on growth in infants: a meta-analysis of randomized controlled trials
European Journal of Nutrition (2019)
-
Nucleotide-mediated SPDEF modulates TFF3-mediated wound healing and intestinal barrier function during the weaning process
Scientific Reports (2018)
-
Purine and pyrimidine salvage pathway in thermophiles: a valuable source of biocatalysts for the industrial production of nucleic acid derivatives
Applied Microbiology and Biotechnology (2018)