Abstract
Objective: To assess whether the addition of viscous fiber at an amount recommended by the US FDA to allow a ‘low saturated fat, cholesterol, soluble fiber and coronary heart disease’, health claim label on a food package (1.7 g psyllium) and/or fat (30 g sunflower oil and 3 g sodium propionate) to a pasta meal would affect gastric emptying, postprandial glucose, insulin and GLP-1 concentrations.
Design: Ten subjects participated in a two-by-two single blind randomized crossover study. Four meals containing 50 g of available carbohydrate were consumed: pasta with or without psyllium enrichment served with a tomato sauce with (520 kcal per meal) and without (240 kcal per meal) fat. Blood samples were taken for 240 min following the meal and all subjects consumed a buffet meal at the end of the study. Gastric empting was measured using the paracetamol absorption test. Blood was analysed for glucose, insulin, GLP-1. Visual analog scales were used to record feelings of hunger, pleasantness and nausea.
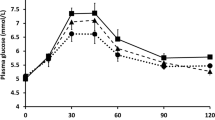
Results: The psyllium-enriched pasta had no significant effect on gastric emptying or the incremental area under the curve (IAUC) for GLP-1, insulin or glucose compared with the control pasta. The addition of polyunsaturated fat and sodium propionate significantly increased the IAUC for GLP-1 (P<0.001), delaying gastric emptying (P<0.002), and decreasing glucose (P<0.002).
Conclusions: A dose of 1.7 g psyllium did not evoke measurable effects on gastric emptying, postprandial GLP-1, insulin or glucose metabolism. However the addition of 30 g of oil and 3 g of sodium propionate to the pasta did reduce gastric emptying, increase GLP-1 and reduce glucose and insulin concentrations. While this short-term study may have implications in terms of reducing the risk of diabetes and improving coronary risk factor profiles the long term effects of these nutrients need to be studied.
Sponsorship: This study was supported by Kellogg Company.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
DeFronzo, RA & Ferrannini, E (1991). Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care, 14, 173–194.
Elahi, D, McAloon-Dyke, M, Fukagawa, NK, Meneilly, GS, Sclater, AL, Minaker, KL, Habener, JF & Anderson, DK (1994). The insulinotrophic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7-37) in normal and diabetic subjects. Regul. Pept., 51, 63–74.
Flint, A, Raben, A, Ersboll, AK, Holst, JJ & Astrup, A (2001). The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int. J. Obes. Relat. Metab. Disord., 25, 781–792.
Frost, G, Keogh, B, Smith, D, Akinsanya, K & Leeds, A (1996). The effect of low-glycemic carbohydrate on insulin and glucose response in vivo and in vitro in patients with coronary heart disease. Metabolism, 45, 669–672.
Frost, G, Leeds, A, Trew, G, Margara, R & Dornhorst, A (1998). Insulin sensitivity in women at risk of coronary heart disease and the effect of a low glycemic diet. Metabolism, 47, 1245–1251.
Gannon, MC, Nuttall, FQ, Westphal, SA & Seaquist, ER (1993). The effect of fat and carbohydrate on plasma glucose, insulin, C-peptide, and triglycerides in normal male patients. J. Am. Coll. Nutr., 12, 36–41.
Heading, RC, Nimmo, J, Prescott, LF & Tothill, P (1973). The dependence of paracetamol absorption on the rate of gastric emptying. Br. J. Pharmac., 47, 415–421.
Hoebler, C, Karinthi, A, Devaux, MF, Guillon, F, Gallant, DJ, Bouchet, B, Melegari, C & Barry, JL (1998). Physical and chemical transformations of cereal food during oral digestion in human subjects. Br. J. Nutr., 80, 429–436.
Jarjis, HA, Blackburn, NA, Redfern, JS & Read, NW (1984). The effect of ispaghula (Fybogel and Metamucil) and guar gum on glucose tolerance in man. Br. J. Nutr., 51, 371–378.
Jenkins, DJ, Wolever, TM, Vuksan, V, Brighenti, F, Cunnane, SC, Rao, AV, Jenkins, AL, Buckley, G, Patten, R & Singer, W et al (1989). Nibbling versus gorging: metabolic advantages of increased meal frequency. New. Engl. J. Med., 321, 929–934.
Kreymann, B, Williams, G, Ghatei, MA & Bloom, SR (1987). Glucagon-like peptide-1 7-36: a physiological increatin in man. Lancet, ii, 1300–1303.
Liljeberg, HG, Akerberg, AK & Bjorck, IM (1999a). Effect of the glycemic index and content of indigestible carbohydrates of cereal-based breakfast meals on glucose tolerance at lunch in healthy subjects. Am. J. Clin. Nutr., 69, 647–655.
Liljeberg, HGM, Akerberg, AKE & Bjork, IME (1999b). Effect of the glycaemic index and content of indigestable carbohydrate of cereal-based breakfast meals on glucose tolerance at lunch in healthy subjects. Am. J. Clin. Nutr., 69, 647–655.
Liljeberg, HGM & Bjorck, IME (1998). Delayed gastric emptying rate may explain improved glycaemia in healthy subjects to a starchy meal with added vinegar. Eur. J. Clin. Nutr., 52, 368–371.
Meneilly, GS, McIntosh, CHS, Pederson, RA, Habener, JF, Gingerich, RG, Egan, BM, Finegood, DT & Elahi, D (2001). Effect of glucagon-like peptide 1 on non-insulin-mediated glucose uptake in the elderly patient with diabetes. Diabetes Care, 24, 1951–1956.
Normand, S, Khalfallah, Y, Louche-Pelissier, C, Pachiaudi, C, Antoine, J-N, Blanc, S, Desage, M, Riou, JP & Laville, M (2001). Influence of dietary fat on postprandial glucose metabolism (exogenous and endogenenous) using intrinsically 13C-enriched durum wheat. Br. J. Nutr., 86, 3–11.
Salmeron, J, Ascherio, A, Rimm, EB, Colditz, GA, Spiegelman, D, Jenkins, DJ, Stampfer, MJ, Wing, AL & Willett, WC (1997a). Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care, 20, 545–550.
Salmeron, J, Manson, JE, Stampfer, MJ, Colditz, GA, Wing, AL & Willett, WC (1997b). Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA, 277, 472–477.
Seidell, JC (2000). Obesity, insulin resistance and diabetes–a worldwide epidemic. Br. J. Nutr., 83, (Suppl 1) S5–S8.
Verdich, C, Toubro, S, Buemann, B, Madsen, J, Holst, JJ & Astrup, A (2001). The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int. J. Obes. Relat. Metab. Disord., 25, 1206–1214.
WHO (2001). Obesity: preventing and managing the global epidemic, WHO/NUT/NCD/98(1) Geneva: WHO
Wolever, TM, Vuksan, V, Eshuis, H, Spadafora, P, Peterson, RD, Chao, ES, Storey, ML & Jenkins, DJ (1991). Effect of method of administration of psyllium on glycemic response and carbohydrate digestibility. J. Am. Coll. Nutr., 10, 364–371.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frost, G., Brynes, A., Dhillo, W. et al. The effects of fiber enrichment of pasta and fat content on gastric emptying, GLP-1, glucose, and insulin responses to a meal. Eur J Clin Nutr 57, 293–298 (2003). https://doi.org/10.1038/sj.ejcn.1601520
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1601520
Keywords
This article is cited by
-
Males and females exhibit similar muscle glycogen recovery with varied recovery food sources
European Journal of Applied Physiology (2020)
-
Influence of the tolerability of vinegar as an oral source of short-chain fatty acids on appetite control and food intake
International Journal of Obesity (2014)
-
Intérêt de la phase postprandiale pour la santé de l’Homme
Obésité (2014)
-
Fiber and Functional Gastrointestinal Disorders
American Journal of Gastroenterology (2013)
-
Incretin hormones and the satiation signal
International Journal of Obesity (2013)