Abstract
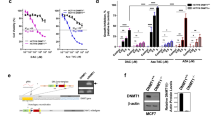
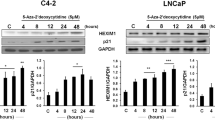
Decitabine (DAC) and 5-azacitidine have recently been approved for the treatment of myelodysplastic syndrome. The pharmacodynamic effects of DAC and 5-azacitidine outside their known activity as inhibitors of DNA methyltransferases (DNMTs) require further investigation. The purpose of this study was to investigate the effect of DAC on the expression of p21WAF1/CIP1, a gene with a putative CpG island surrounding its promoter region. Promoter methylation analysis of p21WAF1/CIP1 in leukemia cells revealed the absence of CpG methylation. However, DAC upregulated p21WAF1/CIP1 expression in a dose-dependent manner (ED50=103.34 nM) and induced G2/M cell cycle arrest in leukemia cells. Sequential application of DAC followed by different histone deacetylase inhibitors induced expression of p21WAF1/CIP1 synergistically. Upregulation of p21WAF1/CIP1 paralleled DAC-induced apoptosis (ED50=153 nM). Low doses of DAC induced γ-H2AX expression (ED50=16.5 nM) and upregulated p21WAF1/CIP1 in congenic HCT 116 colon cancer cells in a DNMT-independent and p53-dependent fashion. Inhibition of p53 transactivation by pifithrin-α or the kinase activity of ATM by either the specific ATM inhibitor KU-5593 or caffeine abrogated p21WAF1/CIP1 upregulation, indicating that DAC upregulation of p21WAF1/CIP1 was p53- and ATM-dependent in leukemia cells. In conclusion, DAC upregulates p21WAF1/CIP1 in DNMT-independent manner via the DNA damage/ATM/p53 axis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Baylin SB, Ohm JE . (2006). Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction? Nat Rev Cancer 6: 107–116.
Biggs JR, Kudlow JE, Kraft AS . (1996). The role of the transcription factor Sp1 in regulating the expression of the WAF1/CIP1 gene in U937 leukemic cells. J Biol Chem 271: 901–906.
Bird AP, Wolffe AP . (1999). Methylation-induced repression—belts, braces, and chromatin. Cell 99: 451–454.
Brakensiek K, Langer F, Kreipe H, Lehmann U . (2005). Absence of p21(CIP 1), p27(KIP 1) and p 57(KIP 2) methylation in MDS and AML. Leuk Res 29: 1357–1360.
Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB . (1999). Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet 21: 103–107.
Chou TC, Talaly P . (1977). A simple generalized equation for the analysis of multiple inhibitions of Michaelis–Menten kinetic systems. J Biol Chem 252: 6438–6442.
Galm O, Herman JG, Baylin SB . (2006). The fundamental role of epigenetics in hematopoietic malignancies. Blood Rev 20: 1–13.
Galm O, Wilop S, Reichelt J, Jost E, Gehbauer G, Herman JG et al. (2004). DNA methylation changes in multiple myeloma. Leukemia 18: 1687–1692.
Gartel AL . (2005). The conflicting roles of the cdk inhibitor p21(CIP1/WAF1) in apoptosis. Leuk Res 29: 1237–1238.
Gartel AL, Radhakrishnan SK . (2005). Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res 65: 3980–3985.
Gartel AL, Tyner AL . (2002). The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther 1: 639–649.
Gore SD, Baylin S, Sugar E, Carraway H, Miller CB, Carducci M et al. (2006). Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res 66: 6361–6369.
Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB . (1996). Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 93: 9821–9826.
Issa JP, Garcia-Manero G, Giles FJ, Mannari R, Thomas D, Faderl S et al. (2004). Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in hematopoietic malignancies. Blood 103: 1635–1640.
Jones PA, Baylin SB . (2002). The fundamental role of epigenetic events in cancer. Nat Rev Genet 3: 415–428.
Ju R, Muller MT . (2003). Histone deacetylase inhibitors activate p21(WAF1) expression via ATM. Cancer Res 63: 2891–2897.
Juttermann R, Li E, Jaenisch R . (1994). Toxicity of 5-aza-2′-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc Natl Acad Sci USA 91: 11797–11801.
Karpf AR, Moore BC, Ririe TO, Jones DA . (2001). Activation of the p53 DNA damage response pathway after inhibition of DNA methyltransferase by 5-aza-2′-deoxycytidine. Mol Pharmacol 59: 751–757.
Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW . (1991). Participation of p53 protein in the cellular response to DNA damage. Cancer Res 51: 6304–6311.
Milutinovic S, Brown SE, Zhuang Q, Szyf M . (2004). DNA methyltransferase 1 knock down induces gene expression by a mechanism independent of DNA methylation and histone deacetylation. J Biol Chem 279: 27915–27927.
Rhee I, Bachman KE, Park BH, Jair KW, Yen RW, Schuebel KE et al. (2002). DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature 416: 552–556.
Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM . (2000). Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem 275: 9390–9395.
Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM et al. (1999). Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res 59: 4375–4382.
Schmelz K, Sattler N, Wagner M, Lubbert M, Dorken B, Tamm I . (2005a). Induction of gene expression by 5-aza-2′-deoxycytidine in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) but not epithelial cells by DNA-methylation-dependent and -independent mechanisms. Leukemia 19: 103–111.
Schmelz K, Wagner M, Dorken B, Tamm I . (2005b). 5-aza-2′-deoxycytidine induces p21WAF expression by demethylation of p73 leading to p53-independent apoptosis in myeloid leukemia. Int J Cancer 114: 683–695.
Scott SA, Dong WF, Ichinohasama R, Hirsch C, Sheridan D, Sanche SE et al. (2006). 5-aza-2′-deoxycytidine (decitabine) can relieve p21WAF1 repression in human acute myeloid leukemia by a mechanism involving release of histone deacetylase 1 (HDAC1) without requiring p21WAF1 promoter demethylation. Leuk Res 30: 69–76.
Tamm I, Wagner M, Schmelz K . (2005). Decitabine activates specific caspases downstream of p73 in myeloid leukemia. Ann Hematol 84(Suppl 13): 47–53.
Taylor WR, Stark GR . (2001). Regulation of the G2/M transition by p53. Oncogene 20: 1803–1815.
Vaute O, Nicolas E, Vandel L, Trouche D . (2002). Functional and physical interaction between the histone methyl transferase Suv39H1 and histone deacetylases. Nucleic Acids Res 30: 475–481.
Wolf D, Rotter V . (1985). Major deletions in the gene encoding the p53 tumor antigen cause lack of p53 expression in HL-60 cells. Proc Natl Acad Sci USA 82: 790–794.
Yoo CB, Jones PA . (2006). Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov 5: 37–50.
Zhu WG, Dai Z, Ding H, Srinivasan K, Hall J, Duan W et al. (2001). Increased expression of unmethylated CDKN2D by 5-aza-2′-deoxycytidine in human lung cancer cells. Oncogene 20: 7787–7796.
Zhu WG, Hileman T, Ke Y, Wang P, Lu S, Duan W et al. (2004). 5-aza-2′-deoxycytidine activates the p53/p21Waf1/Cip1 pathway to inhibit cell proliferation. J Biol Chem 279: 15161–15166.
Acknowledgements
We gratefully acknowledge the gift of congenic HCT116 cells from Dr Bert Vogelstein of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. We thank Drs Robert Arceci, Feyruz Rassool and Michael McDevitt for helpful discussions. This work was supported in part by NCI Grants K24CA111717 and P30 CA06973, translational research award from the Leukemia and Lymphoma Society of America and a grant from the Commonwealth Fund. This work was presented in part at the 2005 meeting of the American Association for Cancer Research (AACR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiemjit, A., Fandy, T., Carraway, H. et al. p21WAF1/CIP1 induction by 5-azacytosine nucleosides requires DNA damage. Oncogene 27, 3615–3623 (2008). https://doi.org/10.1038/sj.onc.1211018
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1211018
Keywords
This article is cited by
-
TET2 and DNMT3A mutations and exceptional response to 4′-thio-2′-deoxycytidine in human solid tumor models
Journal of Hematology & Oncology (2021)
-
Activation of SIRT6 by DNA hypomethylating agents and clinical consequences on combination therapy in leukemia
Scientific Reports (2020)
-
Analysis of the interplay between all-trans retinoic acid and histone deacetylase inhibitors in leukemic cells
Archives of Toxicology (2017)
-
Curcumin and Dimethoxycurcumin Induced Epigenetic Changes in Leukemia Cells
Pharmaceutical Research (2015)
-
A phase I and pharmacodynamic study of the histone deacetylase inhibitor belinostat plus azacitidine in advanced myeloid neoplasia
Investigational New Drugs (2015)