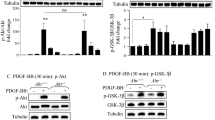
Abstract
The aryl hydrocarbon receptor (AhR) is a transcription factor involved in physiological processes, but also mediates most, if not all, toxic responses to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Activation of the AhR by TCDD leads to its dimerization with aryl hydrocarbon nuclear translocator (ARNT) and transcriptional activation of several phase I and II metabolizing enzymes. However, this classical signalling pathway so far failed to explain the pleiotropic hazardous effects of TCDD, such as developmental toxicity and tumour promotion. Thus, there is an urgent need to define genetic programmes orchestrated by AhR to unravel its role in physiology and toxicology. Here we show that TCDD treatment of rat liver oval cells leads to induction of the transcription factor JunD, resulting in transcriptional upregulation of the proto-oncogene cyclin A which finally triggers a release from contact inhibition. Ectopic expression of cyclin A in confluent cultures overcomes G1 arrest, indicating that increased cyclin A levels are indeed sufficient to bypass contact inhibition. Functional interference with AhR-, but not with ARNT, abolished TCDD-induced increase in JunD and cyclin A and prevented loss of contact inhibition. In summary, we have discovered a novel AhR-dependent and probably ARNT-independent signalling pathway involving JunD and cyclin A, which mediates TCDD-induced deregulation of cell cycle control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Abercrombie M . (1979). Contact inhibition and malignancy. Nature 281: 259–262.
Andersson P, McGuire J, Rubio C, Gradin K, Whitelaw ML, Pettersson S et al. (2002). A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc Natl Acad Sci USA 99: 9990–9995.
Andrews NC, Faller DV . (1991). A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res 19: 2499.
Bock KW, Kohle C . (2005). Ah receptor- and TCDD-mediated liver tumor promotion: clonal selection and expansion of cells evading growth arrest and apoptosis. Biochem Pharmacol 69: 1403–1408.
Casalino L, Bakiri L, Talotta F, Weitzman JB, Fusco A, Yaniv M et al. (2007). Fra-1 promotes growth and survival in RAS-transformed thyroid cells by controlling cyclin A transcription. EMBO J 26: 1878–1890.
Chang C-H, Puga A . (1998). Constitutive activation of the aromatic hydrocarbon receptor. Mol Cell Biol 18: 525–535.
Chramostova K, Vondracek J, Sindlerova L, Vojtesek B, Kozubik A, Machala M . (2004). Polycyclic aromatic hydrocarbons modulate cell proliferation in rat hepatic epithelial stem-like WB-F344 cells. Toxicol Appl Pharmacol 196: 136–148.
Coleman W, McCullough K, Esch G, Faris R, Hixson S, Smith G et al. (1997). Evaluation of the differentiation potential of WB-F344 rat liver epithelial stem-like cells in vivo. Differentiation to hepatocytes after transplantation into dipeptidylpeptidase-IV-deficient rat liver. Am J Pathol 151: 353–359.
De Groot RP, Karperien M, Pals C, Kruijer W . (1991). Characterization of the mouse junD promoter-high basal level activity due to an octamer motif. EMBO J 10: 2523–2532.
Dietrich C, Bartsch T, Schanz F, Oesch F, Wieser R . (1996). p53-dependent cell cycle arrest induced by N-acetyl-L-leucinyl-L-leucinyl-L-norleucinal in platelet-derived growth factor-stimulated human fibroblasts. Proc Natl Acad Sci USA 93: 10815–10819.
Dietrich C, Wallenfang K, Oesch F, Wieser F . (1997). Differences in the mechanisms of growth control in contact-inhibited and serum-deprived human fibroblasts. Oncogene 15: 2743–2747.
Dietrich C, Faust D, Budt S, Moskwa M, Kunz A, Bock K-W et al. (2002). 2,3,7,8-Tetrachlorodibenzo-p-dioxin-dependent release from contact inhibition in WB-F344 cells: involvement of cyclin A. Toxicol Appl Pharmacol 183: 117–126.
Dietrich C, Faust D, Moskwa M, Kunz A, Bock K-W, Oesch F . (2003). TCDD-dependent downregulation of gamma-catenin in rat liver epithelial cells (WB-F344). Int J Cancer 103: 435–439.
Dumble ML, Croager EJ, Yeoh GCT, Quail EA . (2002). Generation and characterization of p53 null transformed hepatic progenitor cells: oval cells give rise to hepatocellular carcinoma. Carcinogenesis 23: 435–445.
Eagle H, Levine E . (1967). Growth regulatory effects of cellular interaction. Nature 213: 1102–1106.
Faust D, Dolado I, Cuadrado A, Oesch F, Weiss C, Nebreda AR et al. (2005). p38alpha MAPK is required for contact inhibition. Oncogene 24: 7941–7945.
Fox TR, Best LL, Goldsworthy SM, Mills JJ, Goldsworthy TL . (1993). Gene expression and cell proliferation in rat liver after 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. Cancer Res 53: 2265–2271.
Hailey JR, Walker NJ, Sells DM, Brix AE, Jokinen MP, Nyska A . (2005). Classification of proliferative hepatocellular lesions in Harlan Sprague–Dawley rats chronically exposed to dioxin-like compounds. Toxicol Pathol 33: 165–174.
Hebert C, Cao Q-L, Birnbaum L . (1990). Inhibition of high-density growth arrest in human squamous carcinoma cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Carcinogenesis 11: 1335–1342.
Heit I, Wieser R, Herget T, Faust D, Borchert-Stuhlträger M, Oesch F et al. (2001). Involvement of protein kinase C delta in contact-dependent inhibition of growth in human and murine fibroblasts. Oncogene 20: 5143–5154.
Hinds PW, Mittnacht S, Dulic V, Arnold A, Reed SI, Weinberg RA . (1992). Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell 70: 993–1006.
Hoffer A, Chang CY, Puga A . (1996). Dioxin induces transcription of fos and jun genes by Ah receptor-dependent and -independent pathways. Toxicol Appl Pharmacol 141: 238–247.
Hushka DR, Greenlee WF . (1995). 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits DNA synthesis in rat primary hepatocytes. Mut Res 333: 89–99.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (1997) In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 69, IARC Press: Lyon, France, pp 1–643.
Kolluri SK, Weiss C, Koff, Göttlicher M . (1999). p27(Kip1) induction and inhibition of proliferation by the intracellular Ah receptor in developing thymus and hepatoma cells. Genes Dev 15: 1742–1753.
Libbrecht L, Desmet V, van Damme B, Roskams T . (2000). The immunohistochemical phenotype of dysplastic foci in human liver: correlation with putative progenitor cells. J Hepatol 33: 76–84.
Ma Q, Whitlock Jr JP . (1996). The aromatic hydrocarbon receptor modulates the Hepa 1c1c7 cell cycle and differentiated state independently of dioxin. Mol Cell Biol 16: 8878–8884.
Marlowe J, Puga A . (2005). Aryl hydrocarbon receptor, cell cycle regulation, toxicity, and tumorigenesis. J Cell Biochem 96: 1174–1184.
Mason GG, Witte AM, Whitelaw ML, Antonsson C, McGuire J, Wilhelmsson A et al. (1994). Purification of the DNA binding form of dioxin receptor. Role of the Arnt cofactor in regulation of dioxin receptor. J Biol Chem 269: 4438–4449.
Moennikes O, Loeppen S, Buchmann A, Andersson P, Ittrich C, Poellinger L et al. (2004). A constitutively active dioxin/aryl hydrocarbon receptor promotes hepatocarcinogenesis in mice. Cancer Res 64: 4707–4710.
Münzel P, Bock-Hennig B, Schieback S, Gschaidmeier H, Beck-Gschaidmeier S, Bock K-W . (1996). Growth modulation of hepatocytes and rat liver epithelial cells (WB-F344) by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Carcinogenesis 17: 197–202.
Musti AM, Treier M, Peverali FA, Bohmann D . (1996). Differential regulation of c-Jun and JunD by ubiquitin-dependent protein degradation. Biol Chem 377: 619–624.
Nebert D, Dalton TP, Okey AB, Gonzales FJ . (2004). Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem 23: 23847–23850.
Pitot HC, Goldsworthy T, Campell HA, Poland A . (1980). Quantitative evaluation of the promotion by 2,3,7,8-tetrachlorodibenzo-p-dioxin of hepatocarcinogenesis from diethyl-nitrosamine. Cancer Res 40: 3616–3620.
Puga A, Nebert DW, Carrier F . (1992). Dioxin induces expression of c-fos and c-jun proto-oncogenes and a large increase in transcription factor AP-1. DNA Cell Biol 11: 269–281.
Shafritz DA, Dabeva MD . (2002). Liver stem cells and model systems for liver repopulation. J Hepatol 36: 552–564.
Shaulian E, Karin M . (2001). AP-1 in cell proliferation and survival. Oncogene 20: 2390–2400.
Shimizu M, Nomura Y, Suzuki H, Ichikawa E, Takeuchi A, Suzuki M et al. (1998). Activation of the rat cyclin A promoter by ATF2 and Jun family members and its suppression by ATF4. Exp Cell Res 239: 93–103.
Steinberg P, Steinbrecher R, Radaeva S, Schirmacher P, Dienes HP, Oesch F et al. (1994). Oval cell lines OC/CDE 6 and OC/CDE 22 give rise to cholangio-cellular and undifferentiated carcinomas after transformation. Lab Invest 71: 700–709.
Stinchcombe S, Buchmann A, Bock K-W, Schwarz M . (1995). Inhibition of apoptosis during 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated tumour promotion in rat liver. Carcinogenesis 16: 1271–1275.
Tsao M-S, Smith J, Nelson K, Grisham J . (1984). A diploid epithelial cell line from normal adult rat liver with phenotypic properties of ‘oval’ cells. Exp Cell Res 154: 38–52.
Tsao M-S, Grisham J . (1987). Hepatocarcinomas, cholangiocarcinomas, and hepatoblastomas produced by chemically transformed cultured rat liver epithelial cells. A light- and electron-microscopic analysis. Am J Pathol 127: 168–181.
Tuomisto J . (2005). Does mechanistic understanding help in risk assessment—the example of dioxins. Toxicol Appl Pharmacol 207: S2–S10.
Vondracek J, Machala M, Bryja V, Chramostova K, Krcmar P, Dietrich C et al. (2005). Aryl hydrocarbon receptor-activating polychlorinated biphenyls and their hydroxylated metabolites induce cell proliferation in contact-inhibited rat liver epithelial cells. Toxicol Sci 83: 53–63.
Weiss C, Kolluri SK, Kiefer F, Göttlicher M . (1996). Complementation of Ah receptor deficiency in hepatoma cells: negative feedback regulation and cell cycle control by the Ah receptor. Exp Cell Res 226: 154–163.
Weiss C, Schneider S, Wagner EF, Zhang X, Seto E, Bohmann D . (2003). JNK phosphorylation relieves HDAC3-dependent suppression of the transcriptional activity of c-Jun. EMBO J 22: 3686–3695.
Weiss C, Faust D, Dürk H, Kolluri SK, Pelzer A, Schneider S et al. (2005). TCDD induces c-jun expression via a novel Ah (dioxin) receptor-mediated p38-MAPK-dependent pathway. Oncogene 24: 4975–4983.
Weitzman JB, Fiette L, Matsuo K, Yaniv M . (2000). JunD protects cells from p53-dependent senescence and apoptosis. Mol Cell 6: 1109–1119.
Wieser R, Faust D, Dietrich C, Oesch F . (1999). p16INK4 mediates contact-inhibition of growth. Oncogene 18: 277–281.
Wörner W, Schrenk D . (1996). Influence of liver tumor promoters on apoptosis in rat hepatocytes induced by 2-acetylaminofluorene, ultraviolet light, or transforming growth factor beta 1. Cancer Res 56: 1272–1278.
Acknowledgements
The expert technical assistance of Sandra Niemann, Andrea Jesenská and Eva Zahradníèková is gratefully acknowledged. We thank Alvaro Puga, Rolf Wenger, Erik Knudsen, Anna Maria Musti and Arata Takeuchi for kindly providing us with various plasmids and Karl-Walter Bock for the WB-F344 cells. We are also indebted to Michael Arand for his helpful support. This work was supported by the Deutsche Forschungsgemeinschaft (Di 793/1-3), the Czech Science Foundation (grant no. 524/06/0517) and the research plan of the Academy of Sciences of the Czech Republic (AV0Z50040702) and by ECNIS (Environmental Cancer Risk, Nutrition and Individual Susceptibility), a network of excellence operating within the European Union 6th Framework Program, Priority 5: ‘Food Quality and Safety’ (Contract no. 513943) and it is part of the MD theses of AR, TF and AM.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Weiss, C., Faust, D., Schreck, I. et al. TCDD deregulates contact inhibition in rat liver oval cells via Ah receptor, JunD and cyclin A. Oncogene 27, 2198–2207 (2008). https://doi.org/10.1038/sj.onc.1210859
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210859
Keywords
This article is cited by
-
Rapid Analysis of Effects of Environmental Toxicants on Tumorigenesis and Inflammation Using a Transgenic Zebrafish Model for Liver Cancer
Marine Biotechnology (2019)
-
Cell–cell contacts protect against t-BuOOH-induced cellular damage and ferroptosis in vitro
Archives of Toxicology (2019)
-
Xenobiotica-metabolizing enzymes in the skin of rat, mouse, pig, guinea pig, man, and in human skin models
Archives of Toxicology (2018)
-
The Brassica-derived phytochemical indolo[3,2-b]carbazole protects against oxidative DNA damage by aryl hydrocarbon receptor activation
Archives of Toxicology (2017)
-
Pure non-dioxin-like PCB congeners suppress induction of AhR-dependent endpoints in rat liver cells
Environmental Science and Pollution Research (2016)