Abstract
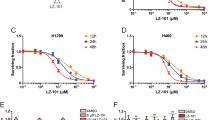
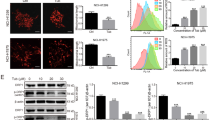
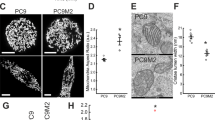
Non-small cell lung carcinomas (NSCLCs) are typically resistant against apoptosis induced by standard chemotherapy. We evaluated the effects of the two potential antitumor agents of the lamellarin class on a highly apoptosis-resistant NSCLC cell line. Both the marine alkaloid lamellarin-D and its synthetic amino derivative PM031379 induced the activation of Bax, the mitochondrial release of cytochrome c and apoptosis-inducing factor (AIF), as well as the activation of caspase-3. However, only PM031379 triggered cell death and sign of nuclear apoptosis coupled to the nuclear translocation of AIF. Depletion of AIF with small interfering RNA or microinjection of a neutralizing anti-AIF antibody largely prevented PM031379-induced cytotoxicity, underscoring the essential contribution of AIF to NSCLC killing. Using NSCLC cells lacking mitochondrial DNA, we showed that the generation of mitochondrial reactive oxygen species (ROS) was crucial for the PM031379-induced translocation of AIF to the nucleus and subsequently cell death. Pretreatment of NSCLC cells with menadione, a mitochondrial ROS generator, was able to restore the deficient chemotherapy-induced apoptosis of NSCLC cells. Altogether, these data suggest that mitochondrial ROS generation is crucial for overriding the chemoresistance of NSCLC cells. Moreover, this study delineates the unique mechanism of action of lamellarins as potential anticancer agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- AIF:
-
apoptosis-inducing factor
- Cyt c:
-
cytochrome c
- HE:
-
hydroethidine
- Lam-D:
-
lamellarin-D
- NAC:
-
N-acetyl cystein
- NSCLC:
-
non-small cell lung carcinoma
- ROS:
-
reactive oxygen species
References
Anderson CP, Tsai J, Chan W, Park CK, Tian L, Lui RM et al. (1997). Buthionine sulphoximine alone and in combination with melphalan (L-PAM) is highly cytotoxic for human neuroblastoma cell lines. Eur J Cancer 33: 2016–2019.
Armand R, Channon JY, Kintner J, White KA, Miselis KA, Perez RP et al. (2004). The effects of ethidium bromide induced loss of mitochondrial DNA on mitochondrial phenotype and ultrastructure in a human leukemia T-cell line (MOLT-4 cells). Toxicol Appl Pharmacol 196: 68–79.
Bailly C . (2004). Lamellarins, from A to Z: a family of anticancer marine pyrrole alkaloids. Curr Med Chem Anticancer Agents 4: 363–378.
Bartling B, Lewensohn R, Zhivotovsky B . (2004). Endogenously released Smac is insufficient to mediate cell death of human lung carcinoma in response to etoposide. Exp Cell Res 298: 83–95.
Bergh J, Nilsson K, Ekman R, Giovanella B . (1985). Establishment and characterization of cell lines from human small cell and large cell carcinomas of the lung. Acta Pathol Microbiol Immunol Scand [A] 93: 133–147.
Broker LE, Huisman C, Span SW, Rodriguez JA, Kruyt FA, Giaccone G . (2004). Cathepsin B mediates caspase-independent cell death induced by microtubule stabilizing agents in non-small cell lung cancer cells. Cancer Res 64: 27–30.
Cande C, Vahsen N, Metivier D, Tourriere H, Chebli K, Garrido C et al. (2004). Regulation of cytoplasmic stress granules by apoptosis-inducing factor. J Cell Sci 117: 4461–4468.
Ekedahl J, Joseph B, Marchetti P, Fauvel H, Formstecher P, Lewensohn R et al. (2003). Heat shock protein 72 does not modulate ionizing radiation-induced apoptosis in U1810 non-small cell lung carcinoma cells. Cancer Biol Ther 2: 663–669.
Engel RH, Evens AM . (2006). Oxidative stress and apoptosis: a new treatment paradigm in cancer. Front Biosci 11: 300–312.
Facompre M, Tardy C, Bal-Mahieu C, Colson P, Perez C, Manzanares I et al. (2003). Lamellarin D: a novel potent inhibitor of topoisomerase I. Cancer Res 63: 7392–7399.
Fennell DA . (2005). Caspase regulation in non-small cell lung cancer and its potential for therapeutic exploitation. Clin Cancer Res 11: 2097–2105.
Ferreira CG, van d V, Span SW, Jonker JM, Postmus PE, Kruyt FA et al. (2001a). Assessment of IAP (inhibitor of apoptosis) proteins as predictors of response to chemotherapy in advanced non-small-cell lung cancer patients. Ann Oncol 12: 799–805.
Ferreira CG, van d V, Span SW, Ludwig I, Smit EF, Kruyt FA et al. (2001b). Expression of X-linked inhibitor of apoptosis as a novel prognostic marker in radically resected non-small cell lung cancer patients. Clin Cancer Res 7: 2468–2474.
Furre IE, Shahzidi S, Luksiene Z, Moller MTN, Borgen E, Morgan J et al. (2005). Targeting PBR by hexaminolevulinate-mediated photodynamic therapy induces apoptosis through translocation of apoptosis-inducing factor in human leukemia cells. Cancer Res 65: 11051–11060.
Gabellini N, Masola V, Quartesan S, Oselladore B, Nobile C, Michelucci R et al. (2006). Increased expression of LGI1 gene triggers growth inhibition and apoptosis of neuroblastoma cells. J Cell Physiol 207: 711–721.
Gallego MA, Joseph B, Hemstrom TH, Tamiji S, Mortier L, Kroemer G et al. (2004). Apoptosis-inducing factor determines the chemoresistance of non-small-cell lung carcinomas. Oncogene 23: 6282–6291.
Galluzzi L, Larochette N, Zamzami N, Kroemer G . (2006). Mitochondria as therapeutic targets for cancer chemotherapy. Oncogene 25: 4812–4830.
Johnstone RW, Ruefli AA, Lowe SW . (2002). Apoptosis: a link between cancer genetics and chemotherapy. Cell 108: 153–164.
Joseph B, Ekedahl J, Lewensohn R, Marchetti P, Formstecher P, Zhivotovsky B . (2001). Defective caspase-3 relocalization in non-small cell lung carcinoma. Oncogene 20: 2877–2888.
Kang YH, Lee E, Choi MK, Ku JL, Kim SH, Park YG et al. (2004a). Role of reactive oxygen species in the induction of apoptosis by alpha-tocopheryl succinate. Int J Cancer 112: 385–392.
Kang YH, Yi MJ, Kim MJ, Park MT, Bae S, Kang CM et al. (2004b). Caspase-independent cell death by arsenic trioxide in human cervical cancer cells: reactive oxygen species-mediated poly(ADP-ribose) polymerase-1 activation signals apoptosis-inducing factor release from mitochondria. Cancer Res 64: 8960–8967.
Kawabe Y, Ochi A . (1991). Programmed cell death and extrathymic reduction of Vb8+ CD4+ T cells in mice tolerant to Staphylococcus aureus anterotoxin B. Nature 349: 245–248.
Kluza J, Gallego MA, Loyens A, Beauvillain JC, Sousa-Faro JM, Cuevas C et al. (2006). Cancer cell mitochondria are direct proapoptotic targets for the marine antitumor drug lamellarin D. Cancer Res 66: 3177–3187.
Krishnaiah P, Reddy VLN, Venkataramana G, Ravinder K, Srinivasulu M, Raju TV et al. (2004). New lamellarin alkaloids from the Indian ascidian Didemnum obscurum and their antioxidant properties. J Nat Prod 67: 1168–1171.
Marchetti P, Susin SA, Decaudin D, Gamen S, Castedo M, Hirsch T et al. (1996a). Apoptosis-associated derangement of mitochondrial function in cells lacking mitochondrial DNA. Cancer Res 56: 2033–2038.
Marchetti P, Zamzami N, Susin SA, Petit PX, Kroemer G . (1996b). Apoptosis of cells lacking mitochondrial DNA. Apoptosis 1: 119–125.
Murahashi H, Azuma H, Zamzami N, Furuya KJ, Ikebuchi K, Yamaguchi M et al. (2003). Possible contribution of apoptosis-inducing factor (AIF) and reactive oxygen species (ROS) to UVB-induced caspase-independent cell death in the T cell line Jurkat. J Leukoc Biol 73: 399–406.
Nyman U, Sobczak-Pluta A, Vlachos P, Perlmann T, Zhivotovsky B, Joseph B . (2005). Full-length p73{alpha} represses drug-induced apoptosis in small cell lung carcinoma cells. J Biol Chem 280: 34159–34169.
O’Connor K, Gill C, Tacke M, Rehmann FJ, Strohfeldt K, Sweeney N et al. (2006). Novel titanocene anti-cancer drugs and their effect on apoptosis and the apoptotic pathway in prostate cancer cells. Apoptosis 11: 1205–1214.
Okouoyo S, Herzer K, Ucur E, Mattern J, Krammer PH, Debatin KM et al. (2004). Rescue of death receptor and mitochondrial apoptosis signaling in resistant human NSCLC in vivo. Int J Cancer 108: 580–587.
Park MT, Kim MJ, Kang YH, Choi SY, Lee JH, Choi JA et al. (2005a). Phytosphingosine in combination with ionizing radiation enhances apoptotic cell death in radiation-resistant cancer cells through ROS-dependent and -independent AIF release. Blood 105: 1724–1733.
Park YC, Jeong JH, Park KJ, Choi HJ, Park YM, Jeong BK et al. (2005b). Sulindac activates nuclear translocation of AIF, DFF40 and endonuclease G but not induces oligonucleosomal DNA fragmentation in HT-29 cells. Life Sci 77: 2059–2070.
Susin SA, Daugas E, Ravagnan L, Samejima K, Zamzami N, Loeffler M et al. (2000). Two distinct pathways leading to nuclear apoptosis. J Exp Med 192: 571–580.
Vahsen N, Cande C, Briere JJ, Benit P, Joza N, Larochette N et al. (2004). AIF deficiency compromises oxidative phosphorylation. EMBO J 23: 4679–4689.
Yang L, Mashima T, Sato S, Mochizuki M, Sakamoto H, Yamori T et al. (2003). Predominant suppression of apoptosome by inhibitor of apoptosis protein in non-small cell lung cancer H460 cells: therapeutic effect of a novel polyarginine-conjugated Smac peptide. Cancer Res 63: 831–837.
Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T et al. (1995). Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med 182: 367–377.
Zhivotovsky B, Orrenius S . (2003). Defects in the apoptotic machinery of cancer cells: role in drug resistance. Semin Cancer Biol 13: 125–134.
Acknowledgements
We thank Pierre Cappy for technical help. In memoriam we like to acknowledge the contribution to this article from Martial Flactif who passed away in July 2006.
This work was supported by grants from INSERM, Université de Lille II, Ligue Nationale contre le Cancer Comité du nord (to PM), Ligue Nationale contre le Cancer (Equipe labellisée) and Institut National contre le Cancer, and European Union ChemoRes (to GK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gallego, MA., Ballot, C., Kluza, J. et al. Overcoming chemoresistance of non-small cell lung carcinoma through restoration of an AIF-dependent apoptotic pathway. Oncogene 27, 1981–1992 (2008). https://doi.org/10.1038/sj.onc.1210833
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210833