Abstract
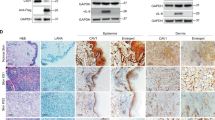
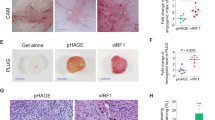
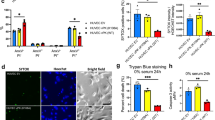
Kaposi's sarcoma (KS) is the most frequent AIDS-associated malignancy, etiologically linked to the infection with the human herpesvirus 8 (HHV-8/KSHV). This member of the γ-herpesviridae family encodes 81 open reading frames, several bearing oncogenic potential. A constitutively active virally encoded G protein–coupled receptor (vGPCR) readily induces KS-like lesions when expressed in endothelial cells in vivo, and unmasks the oncogenic potential of other HHV-8 genes in a paracrine fashion. How vGPCR causes endothelial cell transformation is still not fully understood. Using full-genome microarray analysis we show here that the expression of nuclear factor-κB (NF-κB)-regulated genes is a prominent feature triggered by vGPCR in cells expressing this viral oncogene and in cells exposed to vGPCR-induced secretions, thus mimicking its paracrine effect. Indeed, vGPCR activates the NF-κB pathway potently, and NF-κB activation is a hallmark of both human and experimental KS. Of interest, whereas constitutive NF-κB signaling is not sufficient to promote endothelial cells transformation, NF-κB function is strictly required for vGPCR-induced direct and paracrine neoplasia. Taken together, these results strongly support the role of NF-κB regulated genes in KS pathogenesis, thus providing the rationale for the development of novel mechanism-based therapies for this angioproliferative disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E . (1997). Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385: 347–350.
Asou H, Said JW, Yang R, Munker R, Park DJ, Kamada N et al. (1998). Mechanisms of growth control of Kaposi's sarcoma-associated herpes virus-associated primary effusion lymphoma cells. Blood 91: 2475–2481.
Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS et al. (1998). G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391: 86–89.
Balkwill F . (2004). Cancer and the chemokine network. Nat Rev Cancer 4: 540–550.
Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS . (2004). Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res 64: 5212–5224.
Bower M, Palmieri C, Dhillon T . (2006). AIDS-related malignancies: changing epidemiology and the impact of highly active antiretroviral therapy. Curr Opin Infect Dis 19: 14–19.
Cesarman E, Mesri EA . (2007). Kaposi sarcoma-associated herpesvirus and other viruses in human lymphomagenesis. Curr Top Microbiol Immunol 312: 263–287.
Danial NN, Korsmeyer SJ . (2004). Cell death: critical control points. Cell 116: 205–219.
Dorsam RT, Gutkind JS . (2007). G-protein-coupled receptors and cancer. Nat Rev Cancer 7: 79–94.
Ensoli B, Nakamura S, Salahuddin SZ, Biberfeld P, Larsson L, Beaver B et al. (1989). AIDS-Kaposi's sarcoma-derived cells express cytokines with autocrine and paracrine growth effects. Science 243: 223–226.
Guasparri I, Keller SA, Cesarman E . (2004). KSHV vFLIP is essential for the survival of infected lymphoma cells. J Exp Med 199: 993–1003.
Hamilton TA, Ohmori Y, Tebo J . (2002). Regulation of chemokine expression by antiinflammatory cytokines. Immunol Res 25: 229–245.
Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR et al. (2003). Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem 278: 8508–8515.
Hiscott J, Nguyen TL, Arguello M, Nakhaei P, Paz S . (2006). Manipulation of the nuclear factor-kappaB pathway and the innate immune response by viruses. Oncogene 25: 6844–6867.
Kanegane H, Wakiguchi H, Kanegane C, Kurashige T, Tosato G . (1997). Viral interleukin-10 in chronic active Epstein–Barr virus infection. J Infect Dis 176: 254–257.
Karin M . (2006). Nuclear factor-kappaB in cancer development and progression. Nature 441: 431–436.
Montaner S, Sodhi A, Molinolo A, Bugge TH, Sawai ET, He Y et al. (2003). Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 3: 23–36.
Montaner S, Sodhi A, Pece S, Mesri EA, Gutkind JS . (2001). The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res 61: 2641–2648.
Montaner S, Sodhi A, Ramsdell AK, Martin D, Hu J, Sawai ET et al. (2006). The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor as a therapeutic target for the treatment of Kaposi's sarcoma. Cancer Res 66: 168–174.
Morris K . (2003). Cancer? In Africa? Lancet Oncol 4: 5.
Mutlu AD, Cavallin LE, Vincent L, Chiozzini C, Eroles P, Duran EM et al. (2007). In vivo-restricted and reversible malignancy induced by human herpesvirus-8 KSHV: a cell and animal model of virally induced kaposi's sarcoma. Cancer Cell 11: 245–258.
Pelengaris S, Khan M, Evan G . (2002). c-MYC: more than just a matter of life and death. Nat Rev Cancer 2: 764–776.
Rezza G, Andreoni M, Dorrucci M, Pezzotti P, Monini P, Zerboni R et al. (1999). Human herpesvirus 8 seropositivity and risk of Kaposi's sarcoma and other acquired immunodeficiency syndrome-related diseases. J Natl Cancer Inst 91: 1468–1474.
Schwarz M, Murphy PM . (2001). Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-kappa B and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J Immunol 167: 505–513.
Sciacca FL, Sturzl M, Bussolino F, Sironi M, Brandstetter H, Zietz C et al. (1994). Expression of adhesion molecules, platelet-activating factor, and chemokines by Kaposi's sarcoma cells. J Immunol 153: 4816–4825.
Sodhi A, Montaner S, Gutkind JS . (2004). Viral hijacking of G-protein-coupled-receptor signalling networks. Nat Rev Mol Cell Biol 5: 998–1012.
Yang TY, Chen SC, Leach MW, Manfra D, Homey B, Wiekowski M et al. (2000). Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J Exp Med 191: 445–454.
Acknowledgements
This research was supported by a National Institutes of Health Intramural AIDS Targeted Antiviral Program grant and the National Institute of Dental and Craniofacial Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Martin, D., Galisteo, R., Ji, Y. et al. An NF-κB gene expression signature contributes to Kaposi's sarcoma virus vGPCR-induced direct and paracrine neoplasia. Oncogene 27, 1844–1852 (2008). https://doi.org/10.1038/sj.onc.1210817
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210817
Keywords
This article is cited by
-
A novel role of MNT as a negative regulator of REL and the NF-κB pathway
Oncogenesis (2021)
-
The guanine exchange factor SWAP70 mediates vGPCR-induced endothelial plasticity
Cell Communication and Signaling (2015)
-
Kaposi sarcoma-associated herpesvirus promotes tumorigenesis by modulating the Hippo pathway
Oncogene (2015)
-
Inactivation of a Gαs–PKA tumour suppressor pathway in skin stem cells initiates basal-cell carcinogenesis
Nature Cell Biology (2015)
-
Herpesvirus-encoded GPCRs: neglected players in inflammatory and proliferative diseases?
Nature Reviews Drug Discovery (2014)