Abstract
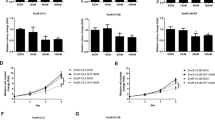
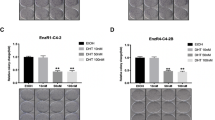
Antiandrogens are initially effective in controlling prostate cancer (CaP), the second most common cancer in men, but resistance, associated with the loss of androgen-regulated cell cycle control, is a major problem. At present there is no effective treatment for androgen-independent prostate cancer (AIPC). Cellular proliferation is driven by cyclin-dependent kinases (CDKs) with kinase inhibitors (for example, p27) applying the breaks. We present the first investigation of the therapeutic potential of CDK inhibitors, using the guanine-based CDK inhibitor NU2058 (CDK2 IC50=17 μ M, CDK1 IC50=26 μ M), in comparison with the antiandrogen bicalutamide (Casodex) in AIPC cells. A panel of AIPC cells was found to be resistant to Casodex-induced growth inhibition, but with the exception of PC3 (GI50=38 μ M) and CWR22Rv1 (GI50=46 μ M) showed similar sensitivity to NU2058 (GI50=10–17 μ M) compared to androgen-sensitive LNCaP cells (GI50=15 μ M). In LNCaP cells and their Casodex-resistant derivative, LNCaP-cdxR, growth inhibition by NU2058 was accompanied by a concentration-dependent increase in p27 levels, reduced CDK2 activity and pRb phosphorylation, a decrease in early gene expression and G1 cell cycle phase arrest in both cell lines. In response to Casodex, there were similar observations in LNCaP cells (GI50=6±3 μ M Casodex) but not in LNCaP-cdxR cells (GI50=24±5 μ M Casodex).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Aleem E, Kiyokawa H, Kaldis P . (2005). Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat Cell Biol 7: 831–836.
Angus SP, Wheeler LJ, Ranmal SA, Zhang X, Markey MP, Mathews CK et al. (2002). Retinoblastoma tumor suppressor targets dNTP metabolism to regulate DNA replication. J Biol Chem 277: 44376–44384.
Arris CE, Boyle FT, Calvert AH, Curtin NJ, Endicott JA, Garman EF et al. (2000). Identification of novel purine and pyrimidine cyclin-dependent kinase inhibitors with distinct molecular interactions and tumor cell growth inhibition profiles. J Med Chem 43: 2797–2804.
Cai D, Latham Jr VM, Zhang X, Shapiro GI . (2006). Combined depletion of cell cycle and transcriptional cyclin-dependent kinase activities induces apoptosis in cancer cells. Cancer Res 66: 9270–9280.
Drees M, Dengler WA, Roth T, Labonte H, Mayo J, Malspeis L et al. (1997). Flavopiridol (L86-8275): selective antitumor activity in vitro and activity in vivo for prostate carcinoma cells. Clin Cancer Res 3: 273–279.
Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA et al. (2005). Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst 97: 1407–1427.
Feldman BJ, Feldman D . (2001). The development of androgen-independent prostate cancer. Nat Rev Cancer 1: 34–45.
Ferrando AA, Balbin M, Pendas AM, Vizoso F, Velasco G, Lopez-Otin C . (1996). Mutational analysis of the human cyclin-dependent kinase inhibitor p27kip1 in primary breast carcinomas. Hum Genet 97: 91–94.
Gregory CW, Johnson Jr RT, Presnell SC, Mohler JL, French FS . (2001). Androgen receptor regulation of G1 cyclin and cyclin-dependent kinase function in the CWR22 human prostate cancer xenograft. J Androl 22: 537–548.
Guo Y, Sklar GN, Borkowski A, Kyprianou N . (1997). Loss of the cyclin-dependent kinase inhibitor p27(Kip1) protein in human prostate cancer correlates with tumor grade. Clin Cancer Res 3: 2269–2274.
Halkidou K, Gnanapragasam VJ, Mehta PB, Logan IR, Brady ME, Cook S et al. (2003). Expression of Tip60, an androgen receptor coactivator, and its role in prostate cancer development. Oncogene 22: 2466–2477.
Hara T, Miyazaki J, Araki H, Yamaoka M, Kanzaki N, Kusaka M et al. (2003). Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res 63: 149–153.
Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC . (1999). Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell 98: 859–869.
Hardcastle IR, Arris CE, Bentley J, Boyle FT, Chen Y, Curtin NJ et al. (2004). N2-substituted O6-cyclohexylmethylguanine derivatives: potent inhibitors of cyclin-dependent kinases 1 and 2. J Med Chem 47: 3710–3722.
Hu B, Mitra J, van den Heuvel S, Enders GH . (2001). S and G2 phase roles for Cdk2 revealed by inducible expression of a dominant-negative mutant in human cells. Mol Cell Biol 21: 2755–2766.
Karan D, Kelly DL, Rizzino A, Lin MF, Batra SK . (2002). Expression profile of differentially-regulated genes during progression of androgen-independent growth in human prostate cancer cells. Carcinogenesis 23: 967–975.
Kawamata N, Morosetti R, Miller CW, Park D, Spirin KS, Nakamaki T et al. (1995). Molecular analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res 55: 2266–2269.
Knillova J, Bouchal J, Hlobilkova A, Strnad M, Kolar Z . (2004). Synergic effects of the cyclin-dependent kinase (CDK) inhibitor olomoucine and androgen-antagonist bicalutamide on prostatic cancer cell lines. Neoplasma 51: 358–367.
Knudsen ES, Wang JY . (1997). Dual mechanisms for the inhibition of E2F binding to RB by cyclin-dependent kinase-mediated RB phosphorylation. Mol Cell Biol 17: 5771–5783.
Kokontis JM, Hsu S, Chuu CP, Dang M, Fukuchi J, Hiipakka RA et al. (2005). Role of androgen receptor in the progression of human prostate tumor cells to androgen independence and insensitivity. Prostate 65: 287–298.
Li Y, Chinni SR, Senderowicz AM, Sarkar FH . (2000). Induction of growth inhibition and apoptosis in prostate cancer cells by flavopiridol. Int J Oncol 17: 755–759.
Macri E, Loda M . (1998). Role of p27 in prostate carcinogenesis. Cancer Metastasis Rev 17: 337–344.
Masiello D, Cheng S, Bubley GJ, Lu ML, Balk SP . (2002). Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J Biol Chem 277: 26321–26326.
Montagnoli A, Fiore F, Eytan E, Carrano AC, Draetta GF, Hershko A et al. (1999). Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev 13: 1181–1189.
Ponce-Castaneda MV, Lee MH, Latres E, Polyak K, Lacombe L, Montgomery K et al. (1995). p27Kip1: chromosomal mapping to 12p12-12p13.1 and absence of mutations in human tumors. Cancer Res 55: 1211–1214.
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D et al. (1990). New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82: 1107–1112.
Taneja SS, Ha S, Garabedian MJ . (2001). Androgen stimulated cellular proliferation in the human prostate cancer cell line LNCaP is associated with reduced retinoblastoma protein expression. J Cell Biochem 84: 188–199.
Tepper CG, Boucher DL, Ryan PE, Ma AH, Xia L, Lee LF et al. (2002). Characterization of a novel androgen receptor mutation in a relapsed CWR22 prostate cancer xenograft and cell line. Cancer Res 62: 6606–6614.
Tetsu O, McCormick F . (2003). Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell 3: 233–245.
Acknowledgements
We thank AICR for financial support, AstraZeneca for Casodex and Professor RJ Griffin (Northern Institute for Cancer Research, School of Natural Sciences-Chemistry, Newcastle University) for NU2058.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rigas, A., Robson, C. & Curtin, N. Therapeutic potential of CDK inhibitor NU2058 in androgen-independent prostate cancer. Oncogene 26, 7611–7619 (2007). https://doi.org/10.1038/sj.onc.1210586
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210586
Keywords
This article is cited by
-
Targeting CDK1 in cancer: mechanisms and implications
npj Precision Oncology (2023)
-
Impact of Androgen Receptor Gene Expression in Gastric Cancer: a Meta-Analysis Based on the GEO Database, TCGA Database, and Literature
Indian Journal of Surgery (2022)
-
Molecular mechanism of the TP53-MDM2-AR-AKT signalling network regulation by USP12
Oncogene (2018)
-
Androgen-regulation of the protein tyrosine phosphatase PTPRR activates ERK1/2 signalling in prostate cancer cells
BMC Cancer (2015)
-
Upregulated FGFR1 expression is associated with the transition of hormone-naive to castrate-resistant prostate cancer
British Journal of Cancer (2011)