Abstract
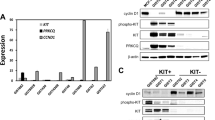
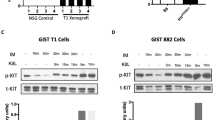
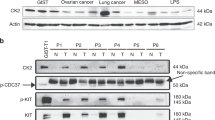
Most gastrointestinal stromal tumors (GISTs) express oncogenic and constitutively active forms of the KIT or platelet-derived growth factor receptor alpha (PDGFRA) receptor tyrosine kinase proteins, and these kinase oncoproteins serve as targets for effective therapies. Given that mutant KIT oncoproteins serve crucial transforming roles in GISTs, we evaluated interactions with the KIT oncoproteins and determined signaling pathways that are dependent on KIT oncogenic activation in GISTs. Tyrosine-phosphorylated KIT oncoproteins interacted with PDGFRA, PDGFRB, phosphatidylinositol 3-kinase (PI3-K) and PKCθ in GIST cells, and these interactions were abolished by KIT inhibition with imatinib or PKC412 or KIT RNAi. Notably, tyrosine-phosphorylated PDGFRA was prominent in frozen GIST tumors expressing KIT oncoproteins, suggesting that KIT-mediated PDGFRA phosphorylation is an efficient and biologically consequential mechanism in GISTs. Activated signaling intermediates were identified by immunoaffinity purification of tyrosine-phosphorylated proteins in GIST cells before and after treatment with KIT inhibitors, and these analyses show that GRB2, SHC, CBL and MAPK activation are largely KIT dependent in GISTs, whereas PI3-K, STAT1 and STAT3 activation are partially KIT dependent. In addition, we found that phosphorylation of several tyrosine kinase proteins – including JAK1 and EPHA4 – did not depend on KIT activation. Likewise, paxillin activation was independent of the KIT oncogenic signal. These studies identify signaling pathways that can provide both KIT-dependent and KIT-independent therapeutic synergies in GIST, and thereby highlight clinical strategies that might consolidate GIST therapeutic response to KIT/PDGFRA inhibition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Blume-Jensen P, Janknecht R, Hunter T . (1998). The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr Biol 8: 779–782.
Debiec-Rychter M, Cools J, Dumez H, Sciot R, Stul M, Mentens N et al. (2005). Mechanisms of resistance to imatinib mesylate in gastrointestinal stromal tumors and activity of the PKC412 inhibitor against imatinib-resistant mutants. Gastroenterology 128: 270–279.
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ et al. (2002). Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347: 472–480.
Duensing A, Joseph NE, Medeiros F, Smith F, Hornick JL, Heinrich MC et al. (2004a). Protein Kinase C theta (PKCtheta) expression and constitutive activation in gastrointestinal stromal tumors (GISTs). Cancer Res 64: 5127–5131.
Duensing A, Medeiros F, McConarty B, Joseph NE, Panigrahy D, Singer S et al. (2004b). Mechanisms of oncogenic KIT signal transduction in primary gastrointestinal stromal tumors (GISTs). Oncogene 23: 3999–4006.
Fabbro D, Ruetz S, Bodis S, Pruschy M, Csermak K, Man A et al. (2000). PKC412 – a protein kinase inhibitor with a broad therapeutic potential. Anticancer Drug Des 15: 17–28.
Hallek M, nhauser-Riedl S, Herbst R, Warmuth M, Winkler A, Kolb HJ et al. (1996). Interaction of the receptor tyrosine kinase p145c-kit with the p210bcr/abl kinase in myeloid cells. Br J Haematol 94: 5–16.
Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N et al. (2003). PDGFRA activating mutations in gastrointestinal stromal tumors. Science 299: 708–710.
Heinrich MC, Rubin BP, Longley BJ, Fletcher JA . (2002). Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum Pathol 33: 484–495.
Herbst R, Shearman MS, Jallal B, Schlessinger J, Ullrich A . (1995). Formation of signal transfer complexes between stem cell and platelet-derived growth factor receptors and SH2 domain proteins in vitro. Biochemistry 34: 5971–5979.
Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S et al. (1998). Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279: 577–580.
Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y et al. (2003). Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology 125: 660–667.
Medeiros F, Corless CL, Duensing A, Hornick JL, Oliveira AM, Heinrich MC et al. (2004). KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol 28: 889–894.
Ning ZQ, Li J, Arceci RJ . (2001). Signal transducer and activator of transcription 3 activation is required for Asp(816) mutant c-Kit-mediated cytokine-independent survival and proliferation in human leukemia cells. Blood 97: 3559–3567.
Otto KG, Jin L, Spencer DM, Blau CA . (2001). Cell proliferation through forced engagement of c-Kit and Flt-3. Blood 97: 3662–3664.
Rubin BP, Singer S, Tsao C, Duensing A, Lux ML, Ruiz R et al. (2001). KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res 61: 8118–8121.
Saucier C, Papavasiliou V, Palazzo A, Naujokas MA, Kremer R, Park M . (2002). Use of signal specific receptor tyrosine kinase oncoproteins reveals that pathways downstream from Grb2 or Shc are sufficient for cell transformation and metastasis. Oncogene 21: 1800–1811.
Sordella R, Bell DW, Haber DA, Settleman J . (2004). Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 305: 1163–1167.
Tauchi T, Feng GS, Marshall MS, Shen R, Mantel C, Pawson T et al. (1994). The ubiquitously expressed Syp phosphatase interacts with c-kit and Grb2 in hematopoietic cells. J Biol Chem 269: 25206–25211.
Tuveson DA, Willis NA, Jacks T, Griffin JD, Singer S, Fletcher CD et al. (2001). STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene 20: 5054–5058.
Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY et al. (2004). Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 364: 1127–1134.
Vultur A, Cao J, Arulanandam R, Turkson J, Jove R, Greer P et al. (2004). Cell-to-cell adhesion modulates Stat3 activity in normal and breast carcinoma cells. Oncogene 23: 2600–2616.
Acknowledgements
We thank Yongchun Zhou for critical review of the article and valuable discussions, Chang-Jie Chen, Anette Duensing and Maureen Thyne for useful discussions and technical assistance. This work was supported by grants from an anonymous donor, the Life Raft Group, Cesarini Team for the Pan-Massachusetts Challenge, the Virginia and Daniel K Ludwig Trust for Cancer Research, the Ronald O Perelman Fund for Cancer Research, the Stutman GIST Cancer Research Fund, the Rubenstein Foundation and Leslie's Links.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Zhu, MJ., Ou, WB., Fletcher, C. et al. KIT oncoprotein interactions in gastrointestinal stromal tumors: therapeutic relevance. Oncogene 26, 6386–6395 (2007). https://doi.org/10.1038/sj.onc.1210464
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210464
Keywords
This article is cited by
-
E3 ubiquitin ligase Atrogin-1 mediates adaptive resistance to KIT-targeted inhibition in gastrointestinal stromal tumor
Oncogene (2021)
-
The V654A second-site KIT mutation increases tumor oncogenesis and STAT activation in a mouse model of gastrointestinal stromal tumor
Oncogene (2020)
-
Cyclin D1 is a mediator of gastrointestinal stromal tumor KIT-independence
Oncogene (2019)
-
Oncogenic signaling by Kit tyrosine kinase occurs selectively on the Golgi apparatus in gastrointestinal stromal tumors
Oncogene (2017)
-
Novel Insights into the Treatment of Imatinib-Resistant Gastrointestinal Stromal Tumors
Targeted Oncology (2017)