Abstract
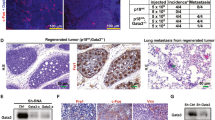
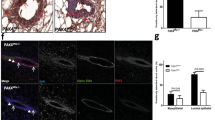
The critical role for GATA family proteins in maintaining the normal (non-transformed) cell state is corroborated by the recent findings of mutations or methylation in GATA genes both in primary cancers and tumor lines including breast. Previously, microarray profiling studies determined that the highest expression of both GATA3 and ESR1 (estrogen receptor α) is seen in tumors associated with the most favorable survival outcomes, whereas the lowest expression of GATA3 is detected in tumor subtypes showing the worst outcomes. At this time, genes and pathways that are regulated by GATA3 in the mammary gland are not well defined. We have previously established a requirement for FOG (Friend Of GATA) cofactors during mouse development. Here we report that in the murine mammary gland Fog2 gene expression is upregulated upon pregnancy and lactation with prominent expression in the epithelial cells of the gland during post-lactational regression. Mammary-specific deletion of Fog2 identified a role for this gene during gland involution; excision of the Fog2 gene leads to the accelerated involution of the gland despite diminished levels of the remodeling enzymes. Importantly, the levels of several genes linked to the control of cancerous transformation in the breast (Esr1, Prg and Foxa1) are significantly reduced upon Fog2 excision. This implicates FOG2 in the maintenance of epithelial cell differentiation in the mammary gland and in performing a protective role in breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Adameyko II, Mudry RE, Houston-Cummings NR, Veselov AP, Gregorio CC, Tevosian SG . (2005). Expression and regulation of mouse SERDIN1, a highly conserved cardiac-specific leucine-rich repeat protein. Dev Dyn 233: 540–552.
Akiyama Y, Watkins N, Suzuki H, Jair KW, van Engeland M, Esteller M et al. (2003). GATA-4 and GATA-5 transcription factor genes and potential downstream antitumor target genes are epigenetically silenced in colorectal and gastric cancer. Mol Cell Biol 23: 8429–8439.
Allar MA, Wood TL . (2004). Expression of the insulin-like growth factor binding proteins during postnatal development of the murine mammary gland. Endocrinology 145: 2467–2477.
Amin DN, Perkins AS, Stern DF . (2004). Gene expression profiling of ErbB receptor and ligand-dependent transcription. Oncogene 23: 1428–1438.
Bertucci F, Borie N, Ginestier C, Groulet A, Charafe-Jauffret E, Adelaide J et al. (2004). Identification and validation of an ERBB2 gene expression signature in breast cancers. Oncogene 23: 2564–2575.
Bertucci F, Houlgatte R, Benziane A, Granjeaud S, Adelaide J, Tagett R et al. (2000). Gene expression profiling of primary breast carcinomas using arrays of candidate genes. Hum Mol Genet 9: 2981–2991.
Booth BW, Smith GH . (2006). Estrogen receptor-alpha and progesterone receptor are expressed in label-retaining mammary epithelial cells that divide asymmetrically and retain their template DNA strands. Breast Cancer Res 8: R49.
Cantor AB, Orkin SH . (2005). Coregulation of GATA factors by the Friend of GATA (FOG) family of multitype zinc finger proteins. Semin Cell Dev Biol 16: 117–128.
Crispino JD . (2005). GATA1 in normal and malignant hematopoiesis. Semin Cell Dev Biol 16: 137–147.
Crispino JD, Lodish MB, MacKay JP, Orkin SH . (1999). Direct association of FOG with GATA-1 is critical for erythroid differentiation and expression of multiple, but not all, GATA-1 target genes. Mol Cell 3: 219–228.
Eeckhoute J, Carroll JS, Geistlinger TR, Torres-Arzayus MI, Brown M . (2006). A cell-type-specific transcriptional network required for estrogen regulation of cyclin D1 and cell cycle progression in breast cancer. Genes Dev 20: 2513–2526.
Gruvberger S, Ringner M, Chen Y, Panavally S, Saal LH, Borg A et al. (2001). Estrogen receptor status in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res 61: 5979–5984.
Han X, Boyd PJ, Colgan S, Madri JA, Haas TL . (2003). Transcriptional up-regulation of endothelial cell matrix metalloproteinase-2 in response to extracellular cues involves GATA-2. J Biol Chem 278: 47785–47791.
Hoch RV, Thompson DA, Baker RJ, Weigel RJ . (1999). GATA-3 is expressed in association with estrogen receptor in breast cancer. Int J Cancer 84: 122–128.
Huggins GS, Wong JY, Hankinson SE, De Vivo I . (2006). GATA5 activation of the progesterone receptor gene promoter in breast cancer cells is influenced by the +331G/A polymorphism. Cancer Res 66: 1384–1390.
Jordan VC . (2004). Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell 5: 207–213.
Kobayashi S, Lackey T, Huang Y, Bisping E, Pu WT, Boxer LM et al. (2006). Transcription factor GATA4 regulates cardiac BCL2 gene expression in vitro and in vivo. FASEB J 20: 800–802.
Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z . (2006). GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 127: 1041–1055.
Lacroix M, Leclercq G . (2004). About GATA3, HNF3A, and XBP1, three genes co-expressed with the oestrogen receptor-alpha gene (ESR1) in breast cancer. Mol Cell Endocrinol 219: 1–7.
Laganiere J, Deblois G, Lefebvre C, Bataille AR, Robert F, Giguere V . (2005). Location analysis of estrogen receptor alpha target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc Natl Acad Sci USA 102: 11651–11656.
Lu JR, McKinsey TA, Xu H, Wang DZ, Richardson JA, Olson EN . (1999). FOG-2, a heart- and brain-enriched cofactor for GATA transcription factors. Mol Cell Biol 19: 4495–4502.
Mallepell S, Krust A, Chambon P, Brisken C . (2006). Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA 103: 2196–2201.
Master SR, Hartman JL, D’Cruz CM, Moody SE, Keiper EA, Ha SI et al. (2002). Functional microarray analysis of mammary organogenesis reveals a developmental role in adaptive thermogenesis. Mol Endocrinol 16: 1185–1203.
Mehra R, Varambally S, Ding L, Shen R, Sabel MS, Ghosh D et al. (2005). Identification of GATA3 as a breast cancer prognostic marker by global gene expression meta-analysis. Cancer Res 65: 11259–11264.
Metcalfe AD, Gilmore A, Klinowska T, Oliver J, Valentijn AJ, Brown R et al. (1999). Developmental regulation of Bcl-2 family protein expression in the involuting mammary gland. J Cell Sci 112: 1771–1783.
Mundschau G, Gurbuxani S, Gamis AS, Greene ME, Arceci RJ, Crispino JD . (2003). Mutagenesis of GATA1 is an initiating event in Down syndrome leukemogenesis. Blood 101: 4298–4300.
Murphy KL, Kittrell FS, Gay JP, Jager R, Medina D, Rosen JM . (1999). Bcl-2 expression delays mammary tumor development in dimethylbenz(α)anthracene-treated transgenic mice. Oncogene 18: 6597–6604.
Parikh P, Palazzo JP, Rose LJ, Daskalakis C, Weigel RJ . (2005). GATA-3 expression as a predictor of hormone response in breast cancer. J Am Coll Surg 200: 705–710.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA et al. (2000). Molecular portraits of human breast tumours. Nature 406: 747–752.
Polyak K . (2006). Pregnancy and breast cancer: the other side of the coin. Cancer Cell 9: 151–153.
Schorr K, Li M, Bar-Peled U, Lewis A, Heredia A, Lewis B et al. (1999). Gain of Bcl-2 is more potent than bax loss in regulating mammary epithelial cell survival in vivo. Cancer Res 59: 2541–2545.
Shen D, Chang HR, Chen Z, He J, Lonsberry V, Elshimali Y et al. (2005). Loss of annexin A1 expression in human breast cancer detected by multiple high-throughput analyses. Biochem Biophys Res Commun 326: 218–227.
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A et al. (2003). Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100: 8418–8423.
Svensson EC, Tufts RL, Polk CE, Leiden JM . (1999). Molecular cloning of FOG-2: a modulator of transcription factor GATA-4 in cardiomyocytes. Proc Natl Acad Sci USA 96: 956–961.
Tevosian SG, Albrecht KH, Crispino JD, Fujiwara Y, Eicher EM, Orkin SH . (2002). Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development 129: 4627–4634.
Tevosian SG, Deconinck AE, Cantor AB, Rieff HI, Fujiwara Y, Corfas G et al. (1999). FOG-2: A novel GATA-family cofactor related to multitype zinc-finger proteins Friend of GATA-1 and U-shaped. Proc Natl Acad Sci USA 96: 950–955.
Tevosian SG, Deconinck AE, Tanaka M, Schinke M, Litovsky SH, Izumo S et al. (2000). FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell 101: 729–739.
Thangaraju M, Rudelius M, Bierie B, Raffeld M, Sharan S, Hennighausen L et al. (2005). C/EBPδ is a crucial regulator of pro-apoptotic gene expression during mammary gland involution. Development 132: 4675–4685.
Thangaraju M, Sharan S, Sterneck E . (2004). Comparison of mammary gland involution between 129S1 and C57BL/6 inbred mouse strains: differential regulation of Bcl21, Trp53, Cebp, and Cebp expression. Oncogene 23: 2548–2553.
Tsang AC, Visvader JE, Turner CA, Fujiwara Y, Yu C, Weiss MJ et al. (1997). FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell 90: 109–119.
Usary J, Llaca V, Karaca G, Presswala S, Karaca M, He X et al. (2004). Mutation of GATA3 in human breast tumors. Oncogene 23: 7669–7678.
van ‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M et al. (2002). Gene expression profiling predicts clinical outcome of breast cancer. Nature 415: 530–536.
van de Rijn M, Perou CM, Tibshirani R, Haas P, Kallioniemi O, Kononen J et al. (2002). Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol 161: 1991–1996.
Wagner KU, McAllister K, Ward T, Davis B, Wiseman R, Hennighausen L . (2001). Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res 10: 545–553.
Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L et al. (1997). Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res 25: 4323–4330.
Wechsler J, Greene M, McDevitt MA, Anastasi J, Karp JE, Le Beau MM et al. (2002). Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat Genet 32: 148–152.
West M, Blanchette C, Dressman H, Huang E, Ishida S, Spang R et al. (2001). Predicting the clinical status of human breast cancer by using gene expression profiles. Proc Natl Acad Sci USA 98: 11462–11467.
Wolf I, Bose S, Williamson EA, Miller CW, Karlan BY, Koeffler HP . (2006). FOXA1: Growth inhibitor and a favorable prognostic factor in human breast cancer. Int J Cancer 120: 1013–1022.
Acknowledgements
We thank Christian Lytle for his advice on qRT-PCR, James DiRenzo for his advice on the whole-mount gland preparations and William Pu for the advice on mouse genotyping. This work was supported by a grant from the Department of Defense Congressionally Mandated Medical Research Program on Breast Cancer (W81XWH-06-1-0394) to SGT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Manuylov, N., Smagulova, F. & Tevosian, S. Fog2 excision in mice leads to premature mammary gland involution and reduced Esr1 gene expression. Oncogene 26, 5204–5213 (2007). https://doi.org/10.1038/sj.onc.1210333
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210333
Keywords
This article is cited by
-
Implications of venous thromboembolism GWAS reported genetic makeup in the clinical outcome of ovarian cancer patients
The Pharmacogenomics Journal (2021)
-
GATA3 Mutations Found in Breast Cancers May Be Associated with Aberrant Nuclear Localization, Reduced Transactivation and Cell Invasiveness
Hormones and Cancer (2013)
-
Estrogen-Induced Aurora Kinase-A (AURKA) Gene Expression is Activated by GATA-3 in Estrogen Receptor-Positive Breast Cancer Cells
Hormones and Cancer (2010)
-
GATA factors in human neuroblastoma: distinctive expression patterns in clinical subtypes
British Journal of Cancer (2009)