Abstract
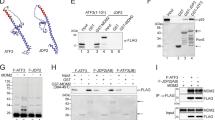
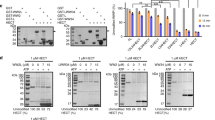
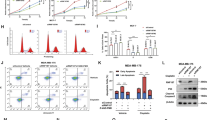
As a key cellular regulatory protein p53 is subject to tight regulation by several E3 ligases. Here, we demonstrate the role of HECT domain E3 ligase, WWP1, in regulating p53 localization and activity. WWP1 associates with p53 and induces p53 ubiquitylation. Unlike other E3 ligases, WWP1 increases p53 stability; inhibition of WWP1 expression or expression of a ligase-mutant form results in decreased p53 expression. WWP1-mediated stabilization of p53 is associated with increased accumulation of p53 in cytoplasm with a concomitant decrease in its transcriptional activities. WWP1 effects are independent of Mdm2 as they are seen in cells lacking Mdm2 expression. Whereas WWP1 limits p53 activity, p53 reduces expression of WWP1, pointing to a possible feedback loop mechanism. Taken together, these findings identify the first instance of a ubiquitin ligase that causes stabilization of p53 while inactivating its transcriptional activities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ashcroft M, Vousden KH . (1999). Regulation of p53 stability. Oncogene 18: 7637–7643.
Bedford MT, Sarbassova D, Xu J, Elder P, Yaffe MB . (2000). A novel pro-Arg motif recognized by WW domains. J Biol Chem 275: 10359–10369.
Brooks CL, Gu W . (2003). Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr Opin Cell Biol 15: 164–171.
Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M . (2006). The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell 124: 601–613.
Chen C, Sun X, Guo P, Dong XY, Sethi P, Cheng X et al. (2005). Human Kruppel-like factor 5 is a target of the E3 ubiquitin ligase WWP1 for proteolysis in epithelial cells. J Biol Chem 280: 41553–41561.
Conkright MD, Wani MA, Lingrel JB . (2001). Lung Kruppel-like factor contains an autoinhibitory domain that regulates its transcriptional activation by binding WWP1, an E3 ubiquitin ligase. J Biol Chem 276: 29299–29306.
Doran D, Wertz I, Shimizu H, Arnott D, Frantz GD, Dowd P et al. (2004). The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature 429: 86–92.
Galinier R, Gout E, Lortat-Jacob H, Wood J, Chroboczek J . (2002). Adenovirus protein involved in virus internalization recruits ubiquitin-protein ligases. Biochemistry 41: 14299–14305.
Harris SL, Levine AJ . (2005). The p53 pathway: positive and negative feedback loops. Oncogene 24: 2899–2908.
Haupt Y, Maya R, Kazaz A, Oren M . (1997). Mdm2 promotes the rapid degradation of p53. Nature 387: 296–299.
Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M . (2002). Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem 277: 3247–3257.
Huang K, Johnson KD, Petcherski AG, Vandergon T, Mosser EA, Copeland NG et al. (2000). A HECT domain ubiquitin ligase closely related to the mammalian protein WWP1 is essential for Caenorhabditis elegans embryogenesis. Gene 252: 137–145.
Ingham RJ, Gish G, Pawson T . (2004). The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene 23: 1972–1984.
Kasanov J, Pirozzi G, Uveges AJ, Kay BK . (2001). Characterizing Class I WW domains defines key specificity determinants and generates mutant domains with novel specificities. Chem Biol 8: 231–241.
Kubbutat MH, Jones SN, Vousden KH . (1997). Regulation of p53 stability by Mdm2. Nature 387: 299–303.
Leng RP, Lin Y, Ma W, Wu H, Lemmers B, Chung S et al. (2003). Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell 112: 779–791.
Li M, Brooks CL, Wu-Baer F, Chen D, Baer R, Gu W . (2003). Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science 302: 1972–1975.
Martin-Serrano J, Eastman SW, Chung W, Bieniasz PD . (2005). HECT ubiquitin ligases link viral and cellular PPXY motifs to the vacuolar protein-sorting pathway. J Cell Biol 168: 89–101.
Moren A, Imamura T, Miyazono K, Heldin CH, Moustakas A . (2005). Degradation of the tumor suppressor Smad4 by WW and HECT domain ubiquitin ligases. J Biol Chem 280: 22115–22123.
Oren M . (2003). Decision making by p53: life, death and cancer. Cell Death and Diff 10: 431–442.
Poyurovsky MV, Prives C . (2006). Unleashing the power of p53: lessons from mice and men. Genes Dev 15: 125–131.
Rossi M, De Laurenzi V, Munarriz E, Green DR, Liu YC, Vousden KH et al. (2005). The ubiquitin-protein ligase Itch regulates p73 stability. EMBO J 24: 836–848.
Sengupta S, Harris CC . (2005). p53: traffic cop at the crossroads of DNA repair and recombination. Nature Rev Mol Cell Biol 6: 44–55.
Shmueli A, Oren M . (2005). Life, death, and ubiquitin: taming the mule. Cell 121: 963–965.
Sudol M, Hunter T . (2000). NeW wrinkles for an old domain. Cell 103: 1001–1004.
Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J et al. (1996). WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J 15: 2371–2380.
Verdecia MA, Bowman ME, Lu KP, Hunter T, Noel JP . (2000). Structural basis for phosphoserine-proline recognition by group IV WW domains. Nat Struct Biol 7: 639–643.
Verdecia MA, Verdecia MA, Joazeiro CA, Wells NJ, Ferrer JL, Bowman ME et al. (2003). Conformational flexibility underlies ubiquitin ligation mediated by the WWP1 HECT domain E3 ligase. Mol Cell 11: 249–259.
Walker KK, Levine AJ . (1996). Identification of a novel p53 functional domain that is necessary for efficient growth suppression. Proc Natl Acad Sci USA 93: 15335–15340.
Wang Z, Fukuda S, Pelus LM . (2004). Survivin regulates the p53 tumor suppressor gene family. Oncogene 23: 8146–8153.
Xu HM, Liao B, Zhang QJ, Wang BB, Li H, Zhong XM et al. (2004). Wwp2, an E3 ubiquitin ligase that targets transcription factor Oct-4 for ubiquitination. J Biol Chem 279: 23495–23503.
Zacchi P, Gostissa M, Uchida T, Salvagno C, Avolio F, Volinia S et al. (2002). The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature 419: 853–857.
Zhang X, Srinivasan SV, Lingrel JB . (2004). WWP1-dependent ubiquitination and degradation of the lung Kruppel-like factor, KLF2. Biochem Biophys Res Commun 316: 139–148.
Zhu J, Zhang S, Jiang J, Chen X . (2000). Definition of the p53 functional domains necessary for inducing apoptosis. J Biol Chem 275: 39927–39934.
Acknowledgements
We thank Marius Sudol and Tony Hunter for providing us with WWP1 reagents, Stephen Jones for p53/Mdm2 DKO cells, and ZQ Pan, J Manfredi and M O'Connell for advice. A Laine performed these studies as part of his work for the MD/PhD program at Mount Sinai School of Medicine, New York, NY. Support by NCI grant CA78419 to ZR is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laine, A., Ronai, Z. Regulation of p53 localization and transcription by the HECT domain E3 ligase WWP1. Oncogene 26, 1477–1483 (2007). https://doi.org/10.1038/sj.onc.1209924
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209924
Keywords
This article is cited by
-
Genome-wide CRISPR screening identifies a role for ARRDC3 in TRP53-mediated responses
Cell Death & Differentiation (2024)
-
The role of NEDD4 related HECT-type E3 ubiquitin ligases in defective autophagy in cancer cells: molecular mechanisms and therapeutic perspectives
Molecular Medicine (2023)
-
WWP1 E3 ligase at the crossroads of health and disease
Cell Death & Disease (2023)
-
The crosstalk between ubiquitin-conjugating enzyme E2Q1 and p53 in colorectal cancer: An in vitro analysis
Medical Oncology (2023)
-
p53 regulation by ubiquitin and ubiquitin-like modifications
Genome Instability & Disease (2022)