Abstract
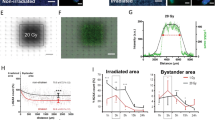
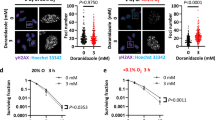
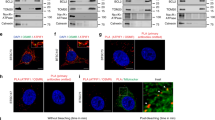
Radiotherapy is an important treatment for patients suffering from high-grade malignant gliomas. Non-targeted (bystander) effects may influence these cells' response to radiation and the investigation of these effects may therefore provide new insights into mechanisms of radiosensitivity and responses to radiotherapy as well as define new targets for therapeutic approaches. Normal primary human astrocytes (NHA) and T98G glioma cells were irradiated with helium ions using the Gray Cancer Institute microbeam facility targeting individual cells. Irradiated NHA and T98G glioma cells generated signals that induced γH2AX foci in neighbouring non-targeted bystander cells up to 48 h after irradiation. γH2AX bystander foci were also observed in co-cultures targeting either NHA or T98G cells and in medium transfer experiments. Dimethyl sulphoxide, Filipin and anti-transforming growth factor (TGF)-beta 1 could suppress γH2AX foci in bystander cells, confirming that reactive oxygen species (ROS) and membrane-mediated signals are involved in the bystander signalling pathways. Also, TGF-beta 1 induced γH2AX in an ROS-dependent manner similar to bystander foci. ROS and membrane signalling-dependent differences in bystander foci induction between T98G glioma cells and normal human astrocytes have been observed. Inhibition of ataxia telangiectasia mutated (ATM) protein and DNA-PK could not suppress the induction of bystander γH2AX foci whereas the mutation of ATM- and rad3-related (ATR) abrogated bystander foci induction. Furthermore, ATR-dependent bystander foci induction was restricted to S-phase cells. These observations may provide additional therapeutic targets for the exploitation of the bystander effect.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Ayache N, Boumediene K, Mathy-Hartert M, Reginster JY, Henrotin Y, Pujol JP . (2002). Expression of TGF-betas and their receptors is differentially modulated by reactive oxygen species and nitric oxide in human articular chondrocytes. Osteoarthritis Cartilage 10: 344–352.
Azzam EI, de Toledo SM, Gooding T, Little JB . (1998). Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res 150: 497–504.
Azzam EI, de Toledo SM, Little JB . (2001). Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to nonirradiated cells. Proc Natl Acad Sci USA 98: 473–478.
Azzam EI, De Toledo SM, Spitz DR, Little JB . (2002). Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res 62: 5436–5442.
Azzam EI, de Toledo SM, Waker AJ, Little JB . (2000). High and low fluences of alpha-particles induce a G1 checkpoint in human diploid fibroblasts. Cancer Res 60: 2623–2631.
Bellocq A, Azoulay E, Marullo S, Flahault A, Fouqueray B, Philippe C et al. (1999). Reactive oxygen and nitrogen intermediates increase transforming growth factor-beta1 release from human epithelial alveolar cells through two different mechanisms. Am J Respir Cell Mol Biol 21: 128–136.
Belyakov OV, Folkard M, Mothersill C, Prise KM, Michael BD . (2002). Bystander-induced apoptosis and premature differentiation in primary urothelial explants after charged particle microbeam irradiation. Radiat Prot Dosimetry 99: 249–251.
Dart DA, Adams KE, Akerman I, Lakin ND . (2004). Recruitment of the cell cycle checkpoint kinase ATR to chromatin during S-phase. J Biol Chem 279: 16433–16440.
Folkard M, Vojnovic B, Hollis KJ, Bowey AG, Watts SJ, Schettino G et al. (1997a). A charged-particle microbeam: II. A single-particle micro-collimation and detection system. Int J Radiat Biol 72: 387–395.
Folkard M, Vojnovic B, Prise KM, Bowey AG, Locke RJ, Schettino G et al. (1997b). A charged-particle microbeam: I. Development of an experimental system for targeting cells individually with counted particles. Int J Radiat Biol 72: 375–385.
Gerashchenko BI, Howell RW . (2003). Cell proximity is a prerequisite for the proliferative response of bystander cells co-cultured with cells irradiated with gamma-rays. Cytometry A 56: 71–80.
Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI et al. (2004). Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res 64: 9152–9159.
Hsu HY, Wen MH . (2002). Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem 277: 22131–22139.
Hu B, Han W, Wu L, Feng H, Liu X, Zhang L et al. (2005). In situ visualization of DSBs to assess the extranuclear/extracellular effects induced by low-dose alpha-particle irradiation. Radiat Res 164: 286–291.
Hwang YS, Jeong M, Park JS, Kim MH, Lee DB, Shin BA et al. (2004). Interleukin-1beta stimulates IL-8 expression through MAP kinase and ROS signaling in human gastric carcinoma cells. Oncogene 23: 6603–6611.
Kashino G, Prise KM, Schettino G, Folkard M, Vojnovic B, Michael BD et al. (2004). Evidence for induction of DNA double strand breaks in the bystander response to targeted soft X-rays in CHO cells. Mutat Res 556: 209–215.
Kosmidou I, Vassilakopoulos T, Xagorari A, Zakynthinos S, Papapetropoulos A, Roussos C . (2002). Production of interleukin-6 by skeletal myotubes: role of reactive oxygen species. Am J Respir Cell Mol Biol 26: 587–593.
Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkelsen RB . (2001). Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res 61: 3894–3901.
Liao H, Winkfein RJ, Mack G, Rattner JB, Yen TJ . (1995). CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J Cell Biol 130: 507–518.
Lyng FM, Seymour CB, Mothersill C . (2002). Initiation of apoptosis in cells exposed to medium from the progeny of irradiated cells: a possible mechanism for bystander-induced genomic instability? Radiat Res 157: 365–370.
Maguire P, Mothersill C, Seymour C, Lyng FM . (2005). Medium from irradiated cells induces dose-dependent mitochondrial changes and BCL2 responses in unirradiated human keratinocytes. Radiat Res 163: 384–390.
Mitchell SA, Randers-Pehrson G, Brenner DJ, Hall EJ . (2004). The bystander response in C3H 10T1/2 cells: the influence of cell-to-cell contact. Radiat Res 161: 397–401.
Morgan WF . (2003). Is there a common mechanism underlying genomic instability, bystander effects and other nontargeted effects of exposure to ionizing radiation? Oncogene 22: 7094–7099.
Mothersill C, Seymour RJ, Seymour CB . (2004a). Bystander effects in repair-deficient cell lines. Radiat Res 161: 256–263.
Mothersill CE, Moriarty MJ, Seymour CB . (2004b). Radiotherapy and the potential exploitation of bystander effects. Int J Radiat Oncol Biol Phys 58: 575–579.
Nagasawa H, Huo L, Little JB . (2003). Increased bystander mutagenic effect in DNA double-strand break repair-deficient mammalian cells. Int J Radiat Biol 79: 35–41.
Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM . (2000). A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol 10: 886–895.
Prise KM, Schettino G, Folkard M, Held KD . (2005). New insights on cell death from radiation exposure. Lancet Oncol 6: 520–528.
Rogakou EP, Boon C, Redon C, Bonner WM . (1999). Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol 146: 905–916.
Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM . (1998). DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273: 5858–5868.
Rothkamm K, Lobrich M . (2003). Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci USA 100: 5057–5062.
Ryan KA, Smith Jr MF, Sanders MK, Ernst PB . (2004). Reactive oxygen and nitrogen species differentially regulate Toll-like receptor 4-mediated activation of NF-kappa B and interleukin-8 expression. Infect Immun 72: 2123–2130.
Sawant SG, Randers-Pehrson G, Geard CR, Brenner DJ, Hall EJ . (2001). The bystander effect in radiation oncogenesis: I. Transformation in C3H 10T1/2 cells in vitro can be initiated in the unirradiated neighbors of irradiated cells. Radiat Res 155: 397–401.
Schultz LB, Chehab NH, Malikzay A, Halazonetis TD . (2000). p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J Cell Biol 151: 1381–1390.
Shao C, Folkard M, Michael BD, Prise KM . (2004). Targeted cytoplasmic irradiation induces bystander responses. Proc Natl Acad Sci USA 101: 13495–13500.
Shao C, Folkard M, Michael BD, Prise KM . (2005). Bystander signaling between glioma cells and fibroblasts targeted with counted particles. Int J Cancer 116: 45–51.
Shao C, Furusawa Y, Aoki M, Ando K . (2003a). Role of gap junctional intercellular communication in radiation-induced bystander effects in human fibroblasts. Radiat Res 160: 318–323.
Shao C, Lyng F, Folkard M, Prise KM . (2006). Calcium fluxes modulate the radiation induced bystander response in targeted glioma and fibroblast cells. Radiat Res (in press).
Shao C, Stewart V, Folkard M, Michael BD, Prise KM . (2003b). Nitric oxide-mediated signaling in the bystander response of individually targeted glioma cells. Cancer Res 63: 8437–8442.
Sokolov MV, Smilenov LB, Hall EJ, Panyutin IG, Bonner WM, Sedelnikova OA . (2005). Ionizing radiation induces DNA double-strand breaks in bystander primary human fibroblasts. Oncogene 24: 7257–7265.
Souhami R, Tobias J . (2005). Cancer and Its Management, 5th edn. Blackwell Pub: Oxford, UK.
Stiff T, O'Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA . (2004). ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res 64: 2390–2396.
Thannickal VJ, Fanburg BL . (1995). Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem 270: 30334–30338.
Veuger SJ, Curtin NJ, Richardson CJ, Smith GC, Durkacz BW . (2003). Radiosensitization and DNA repair inhibition by the combined use of novel inhibitors of DNA-dependent protein kinase and poly(ADP-ribose) polymerase-1. Cancer Res 63: 6008–6015.
Wang H, Wang M, Bocker W, Iliakis G . (2005). Complex H2AX phosphorylation patterns by multiple kinases including ATM and DNA-PK in human cells exposed to ionizing radiation and treated with kinase inhibitors. J Cell Physiol 202: 492–502.
Ward IM, Chen J . (2001). Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem 276: 47759–47762.
Yang H, Asaad N, Held KD . (2005). Medium-mediated intercellular communication is involved in bystander responses of X-ray-irradiated normal human fibroblasts. Oncogene 24: 2096–2103.
Zhou H, Randers-Pehrson G, Waldren CA, Vannais D, Hall EJ, Hei TK . (2000). Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc Natl Acad Sci USA 97: 2099–2104.
Acknowledgements
We are grateful to Stuart Gilchrist and Bob Sunderland for providing excellent technical support for the microbeam irradiation. Graeme Smith, KuDos Pharmaceuticals, Cambridge, UK, kindly provided the ATM inhibitor KU-55933 and the DNA-PK inhibitor NU 7026. Penny Jeggo, University of Sussex, UK, kindly provided ATR Seckel fibroblasts (F02/98 htert) and suitable control cells (48BR htert). This study was funded by a grant from the James S McDonnell Foundation to SC Short, M Folkard and KM Prise with additional support from the Gray Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burdak-Rothkamm, S., Short, S., Folkard, M. et al. ATR-dependent radiation-induced γH2AX foci in bystander primary human astrocytes and glioma cells. Oncogene 26, 993–1002 (2007). https://doi.org/10.1038/sj.onc.1209863
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209863
Keywords
This article is cited by
-
The cross-talk between Bax, Bcl2, caspases, and DNA damage in bystander HepG2 cells is regulated by γ-radiation dose and time of conditioned media transfer
Apoptosis (2022)
-
Reverse transcriptase inhibition potentiates target therapy in BRAF-mutant melanomas: effects on cell proliferation, apoptosis, DNA-damage, ROS induction and mitochondrial membrane depolarization
Cell Communication and Signaling (2020)
-
Integrated Modelling of Cell Responses after Irradiation for DNA-Targeted Effects and Non-Targeted Effects
Scientific Reports (2018)
-
Radiation, inflammation and the immune response in cancer
Mammalian Genome (2018)
-
Radiation quality-dependence of bystander effect in unirradiated fibroblasts is associated with TGF-β1-Smad2 pathway and miR-21 in irradiated keratinocytes
Scientific Reports (2015)