Abstract
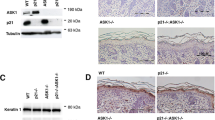
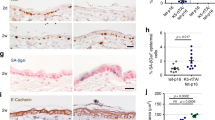
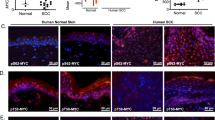
Recent studies have identified roles for C/EBPβ in cellular survival and tumorigenesis, however, the mechanisms through which C/EBPβ regulates these processes are not fully understood. Previously, we demonstrated that C/EBPβ−/− mice are resistant to carcinogen-induced skin tumorigenesis and in response to topical carcinogen treatment display a 17-fold increase in keratinocyte apoptosis compared to wild-type mice. Here, we have investigated the mechanisms through which C/EBPβ regulates apoptosis in response to carcinogenic stress. Analysis of carcinogen-treated C/EBPβ−/− mouse skin revealed a striking increase in the number of p53 immunopositive keratinocytes in the epidermis of C/EBPβ−/− mice compared to wild-type mice and this increase was temporally associated with a concomitant anomalous increase in apoptosis. The increased levels of p53 were functional as Mdm2, Bcl-2, C/EBPα and p21 were differentially regulated in the epidermis of carcinogen-treated C/EBPβ−/− mice. The increase in p53 protein was not associated with an increase in p53 mRNA levels. To determine whether p53 is required for the increased apoptosis in C/EBPβ−/− mice, C/EBPβ/p53 compound knockout mice were generated. Carcinogen-treated C/EBPβ/p53 compound knockout mice did not display increased apoptosis demonstrating p53 is required for the proapoptotic phenotype in C/EBPβ−/− mice. Our results demonstrate that altered keratinocyte survival in C/EBPβ−/− mice results from aberrant regulation of p53 protein and function and indicate C/EBPβ has a role in the negative regulation of p53 protein levels in response to carcinogen-induced stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Appella E, Anderson CW . (2001). Eur J Biochem 268: 2764–2772.
Brooks CL, Gu W . (2006). Mol Cell 21 (3): 307–315.
Buck M, Poli V, Hunter T, Chojkier M . (2001). Mol Cell 8: 807–816.
Bundy LM, Sealy L . (2003). Oncogene 22: 869–883.
Burkart AD, Mukherjee A, Sterneck E, Johnson PF, Mayo KE . (2005). Endocrinology 146: 1909–1921.
Corbi AL, Jensen UB, Watt FM . (2000). FEBS Lett 474: 201–207.
Di-Poi N, Desvergne B, Michalik L, Wahli W. (2005). J Biol Chem 280: 38700–38710.
Freedman DA, Levine AJ . (1998). Mol Cell Biol 18: 7288–7293.
Harris SL, Levine AJ . (2005). Oncogene 24: 2899–2908.
Honda R, Tanaka H, Yasuda H . (1997). FEBS Lett 420: 25–27.
Ito A, Kawaguchi Y, Lai CH, Kovacs JJ, Higashimoto Y, Appella E et al. (2002). Embo J 21: 6236–6245.
Jackson MW, Berberich SJ . (2000). Mol Cell Biol 20: 1001–1007.
Kamijo T, Weber JD, Zambetti G, Zindy F, Roussel MF, Sherr CJ . (1998). Proc Natl Acad Sci USA 95: 8292–8297.
Lamb J, Ramaswamy S, Ford HL, Contreras B, Martinez RV, Kittrell FS et al. (2003). Cell 114: 323–334.
Li W, Kessler P, Yeger H, Alami J, Reeve AE, Heathcott R et al. (2005). Cancer Res 65: 2592–2601.
Maya R, Balass M, Kim ST, Shkedy D, Leal JF, Shifman O et al. (2001). Genes Dev 15: 1067–1077.
Maytin EV, Habener JF . (1998). J Invest Dermatol 110: 238–246.
Oh HS, Smart RC . (1998). J Invest Dermatol 110: 939–945.
Pomerantz J, Schreiber-Agus N, Liegeois NJ, Silverman A, Alland L, Chin L et al. (1998). Cell 92: 713–723.
Ramji DP, Foka P . (2002). Biochem J 365 (Part 3): 561–575.
Rask K, Thorn M, Poten F, Kraaz W, Sundfeldt K, Hedin L et al. (2000). Int J Cancer 86: 337–343.
Sankpal NV, Mayo MW, Powell SM . (2005). Biochim Biophys Acta 1728: 1–10.
Sebastian T, Malik R, Thomas S, Sage J, Johnson PF . (2005). EMBO J 24: 3301–3312.
Shieh SY, Taya Y, Prives C . (1999). EMBO J 18: 1815–1823.
Shuman JD, Sebastian T, Kaldis P, Copeland TD, Zhu S, Smart RC et al. (2004). Mol Cell Biol 24: 7380–7391.
Sterneck E, Tessarollo L, Johnson PF . (1997). Genes Dev 11: 2153–2162.
Sterneck E, Zhu S, Ramirez A, Jorcano JL, Smart RC . (2005). Oncogene 25: 1272–12776.
Sundfeldt K, Ivarsson K, Carlsson M, Enerback S, Janson PO, Brannstrom M et al. (1999). Br J Cancer 79: 1240–1248.
Tao W, Levine AJ . (1999). Proc Natl Acad Sci USA 96: 6937–6941.
Vogelstein B, Lane D, Levine AJ . (2000). Nature 408: 307–310.
Vousden KH, Lu X . (2002). Nat Rev Cancer 2: 594–604.
Wessells J, Yakar S, Johnson PF . (2004). Mol Cell Biol 24: 3238–3250.
Zhang Y, Xiong Y, Yarbrough WG . (1998). Cell 92: 725–734.
Zhu S, Oh HS, Shim M, Sterneck E, Johnson PF, Smart RC . (1999). Mol Cell Biol 19: 7181–7190.
Zhu S, Yoon K, Sterneck E, Johnson PF, Smart RC . (2002). Proc Natl Acad Sci USA 99: 207–212.
Acknowledgements
We would like to thank Dr Cavell Brownie for help with the statistical analysis. This work was supported by a grant CA46637 from the National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, K., Zhu, S., Ewing, S. et al. Decreased survival of C/EBPβ-deficient keratinocytes is due to aberrant regulation of p53 levels and function. Oncogene 26, 360–367 (2007). https://doi.org/10.1038/sj.onc.1209797
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209797
Keywords
This article is cited by
-
C/EBPβ deletion in oncogenic Ras skin tumors is a synthetic lethal event
Cell Death & Disease (2018)
-
CCAAT/enhancer binding protein beta protects muscle satellite cells from apoptosis after injury and in cancer cachexia
Cell Death & Disease (2016)
-
LIP expression is regulated by IGF-1R signaling and participates in suppression of anoikis
Molecular Cancer (2011)
-
C/EBPβ represses p53 to promote cell survival downstream of DNA damage independent of oncogenic Ras and p19Arf
Cell Death & Differentiation (2008)