Abstract
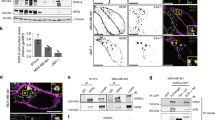
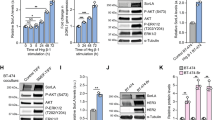
Although combinatorial signaling through the ErbB network is implicated in certain types of human cancer, the specifics of how particular receptors contribute to the transformed phenotype are not well understood. The goal of this study was to identify epidermal growth factor (EGF) receptor-dependent cell signaling abnormalities specifically associated with mutations in a previously described 679-LL lysosomal sorting signal, which restrict ligand-dependent receptor downregulation by promoting recycling. Importantly, the 679-LL signal is not conserved in any of the other members of the ErbB receptor family suggesting its physiological function may be tightly regulated during EGF receptor-dependent signaling. Our data indicate that cells expressing receptors with an inactive 679-AA signal are rapidly transported to Rab4+ early endosomes after they are internalized in contrast to wild-type receptors that are localized to early endocytic antigen 1 (EEA1)+ early endosomes. Divergent trafficking in early endosomes is associated with prolonged activation of p44/42 mitogen-activated protein kinases (MAPK) but not Akt. Gab1 appears to be the critical signaling molecule facilitating prolonged MAPK signaling, and activated Gab1 is recruited to early endosomes in 679-AA receptor-expressing cells. Activated Gab1 is also recruited to early endosomes in breast cancer cells characterized by high levels of EGF receptor-ErbB2 heterodimers, suggesting 679-AA expressing cells recapitulate certain aspects of EGF receptor signaling and transformation by activated ErbB2. Phosphatidylinositol 3-kinase (PI3K)-dependent membrane translocation known to be important for maintaining Gab1 activity in other settings was dispensable. We conclude that 679-LL has dual functions in EGF receptor trafficking and threshold signaling through a subset of signaling molecules including p44/42 MAPK and Gab1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Abbreviations
- EEA1:
-
early endocytic antigen 1
- EGFR:
-
epidermal growth factor receptor
- MAPK:
-
mitogen-activated protein kinases
- MBD:
-
Met binding domain
- PH:
-
pleckstrin homology
- PI3K:
-
phosphatidylinositol 3-kinase
References
Alroy I, Yarden Y . (1997). FEBS Lett 410: 83–86.
Baulida J, Kraus MH, Alimandi M, Fiore PPD, Carpenter G . (1996). J Biol Chem 271: 5251–5257.
Bonifacino J, Traub L . (2003). Annu Rev Biochem 72: 395–440.
Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B et al. (1992). Cell 70: 715–728.
Cai T, Nishida K, Hirano T, Khavari P . (2002). J Cell Biol 159: 103–112.
Chakraborty A . (2002). Hosp Med 63: 743–745.
Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M . (1990). Cell 62: 317–329.
Chen X, Wang Z . (2001). EMBO Rep 2: 842–849.
Christoforidis S, McBride H, Burgoyne R, Zerial M . (1999). Nature 397: 621–625.
Citri A, Skaria KB, Yarden Y . (2003). Exp Cell Res 284: 54–65.
Crooks D, Kil SJ, McCaffery JM, Carlin C . (2000). Mol Biol Cell 11: 3559–3572.
Cunnick J, Dorsey J, Munoz-Antonia T, Mei L, Wu J . (2000). J Biol Chem 275: 13842–13848.
Cunnick JM, Mei L, Doupnik CA, Wu J . (2001). J Biol Chem 276: 24380–24387.
Daro E, van der Sluijs P, Galli T, Mellman I . (1996). Proc Natl Acad Sci USA 93: 9559–9564.
de Renzis S, Sonnichsen B, Zerial M . (2002). Nat Cell Biol 4: 124–133.
Downward J . (2004). Semin Cell Dev Biol 15: 177–182.
Feng Y, Press B, Wandinger-Ness A . (1995). J Cell Biol 131: 1435–1452.
Fragale A, Tartaglia M, Wu J, Gelb BD . (2004). Hum Mutat 23: 267–277.
Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier JM et al. (2000). EMBO J 19: 4577–4588.
Gu H, Neel BG . (2003). Trends Cell Biol 13: 122–126.
Gual P, Giordano S, Williams T, Rocchi S, Van Obberghen E, Comoglio P . (2000). Oncogene 19: 1509–1518.
Harlow E, Lane D . (1988) In: Antibodies: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory, pp 139–242.
Hendriks BS, Opresko LK, Wiley HS, Lauffenburger DA . (2003). J Biol Chem 278: 23343–23351.
Holgado-Madruga M, Emlet DR, Moscatello DK, Godwin AK, Wong AJ . (1996). Nature 379: 560–564.
Holgado-Madruga M, Moscatello D, Emlet D, Dieterich R, Wong A . (1997). Proc Natl Acad Sci USA 94: 12419–12424.
Isakoff SJ, Cardozo T, Andreev J, Li Z, Ferguson KM, Abagyan R et al. (1998). EMBO J 17: 5374–5387.
Itoh M, Yoshida Y, Nishida K, Narimatsu M, Hibi M, Hirano T . (2000). Mol Cell Biol 20: 3695–3704.
Jones SM, Foreman SK, Shank BB, Kurten RC . (2002). Am J Physiol Cell Physiol 282: C420–C433.
Kameda H, Risinger JI, Han BB, Baek SJ, Barrett JC, Abe T et al. (2001). Mol Cell Biol 21: 6895–6905.
Karunagaran D, Tzahar E, Beerli RR, Chen X, Graus-Porta D, Ratzkin BJ et al. (1996). EMBO J 15: 254–264.
Kil S, Carlin C . (2000). J Cell Physiol 185: 47–60.
Kil SJ, Hobert ME, Carlin C . (1999). J Biol Chem 274: 3141–3150.
Klausner RD, Van Renswoude J, Ashwell G, Kempf C, Schecter AN, Dean A et al. (1983). J Biol Chem 258: 4715–4724.
Krendel M, Mooseker MS . (2005). Physiology 20: 239–251.
Kurten RC, Cadena DL, Gill GN . (1996). Science 272: 1008–1110.
Laemmli UK . (1970). Nature 227: 680–685.
Lamb J, Ray F, Ward J, Kushner J, Kaplan J . (1983). J Biol Chem 258: 8751–8758.
Lenferink A, Pinkas-Kramarski R, van de Poll M, van Vugt M, Klapper L, Tzahar E et al. (1998). EMBO J 17: 3385–3397.
Lippincott-Schwartz J, Fambrough DM . (1987). Cell 49: 669–677.
Lock L, Royal I, Naujokas M, Park M . (2000). J Biol Chem 275: 31536–31545.
Margolis B, Skolnik E . (1994). J Am Soc Nephrol 5: 1288–1299.
Marmor MD, Yarden Y . (2004). Oncogene 15: 2057–2070.
Maroun CR, Moscatello DK, Naujokas MA, Holgado-Madruga M, Wong AJ, Park M . (1999). J Biol Chem 274: 31719–31726.
Miaczynska M, Pelkmans L, Zerial M . (2004). Curr Opin Cell Biol 16: 400–406.
Nakanishi S, Kakita S, Takahashi I, Kawahara K, Tsukuda E, Sano T et al. (1992). J Biol Chem 267: 2157–2163.
Olayioye MA, Graus-Porta D, Beerli RR, Rohrer J, Gay B, Hynes NE . (1998). Mol Cell Biol 18: 5042–5051.
Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K et al. (2001). Endocr Rev 22: 153–183.
Pruss RM, Herschman HR . (1977). Proc Natl Acad Sci USA 74: 3918–3921.
Rodrigues GA, Falasca M, Zhang Z, Ong SH, Schlessinger J . (2000). Mol Cell Biol 20: 1448–1459.
Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J et al. (1998). Nature 394: 494–498.
Simonsen A, Wurmser AE, Emr SD, Stenmark H . (2001). Curr Opin Cell Biol 13: 485–492.
Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M . (2000). J Cell Biol 149: 901–914.
Sorkin A, Von Zastrow M . (2002). Nat Rev Mol Cell Biol 3: 600–614.
Takahashi-Tezuka M, Yoshida Y, Fukada T, Ohtani T, Yamanaka Y, Nishida K et al. (1998). Mol Cell Biol 18: 4109–4117.
Towbin H, Staehelin T, Gordon J . (1979). Proc Natl Acad Sci USA 76: 4350–4354.
Tsacoumangos A, Kil SJ, Ma L, Sonnichsen FD, Carlin C . (2005). J Cell Sci 118: 3959–3971.
van der Sluijs SP, Hull M, Webster P, Male P, Goud B, Mellman I . (1992). Cell 70: 729–740.
Vitale G, Rybin V, Christoforidis S, Thornqvist P, McCaffrey M, Stenmark H et al. (1998). EMBO J 17: 1941–1951.
Wang Z, Zhang L, Yeung TK, Chen X . (1999). Mol Biol Cell 10: 1621–1636.
Waterman H, Katz M, Rubin C, Shtiegman K, Lavi S, Elson A et al. (2002). EMBO J 21: 303–313.
Waterman H, Yarden Y . (2001). FEBS Lett 490: 142–152.
Weidner KM, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W . (1996). Nature 173: 384–386.
Weiel JE, Hamilton TA . (1984). Biochem Biophys Res Comm 119: 598–603.
Wiley HS . (2003). Exp Cell Res 284: 78–88.
Wiley HS, Burke PM . (2001). Traffic 2: 12–18.
Wilson JM, de Hoop M, Zorzi N, Toh BH, Dotti CG, Parton RG . (2000). Mol Biol Cell 11: 2657–2671.
Wolf I, Jenkins B, Liu Y, Seiffert M, Custodio J, Young P et al. (2002). Mol Cell Biol 22: 231–244.
Yamasaki S, Nishida K, Yoshida Y, Itoh M, Hibi M, Hirano T . (2003). Oncogene 22: 1546–1556.
Yarden Y, Sliwkowski MX . (2001). Nat Rev Mol Cell Biol 2: 127–137.
Zerial M, McBride H . (2001). Nat Rev 2: 107–118.
Zhao C, Yu D, Shen R, Feng G . (1999). J Biol Chem 274: 19649–19654.
Acknowledgements
We wish to thank other members of the Carlin laboratory for lively discussions during preparation of this manuscript. This work was supported by Public Health Service grant RO1 GM64243 to CC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kostenko, O., Tsacoumangos, A., Crooks, D. et al. Gab1 signaling is regulated by EGF receptor sorting in early endosomes. Oncogene 25, 6604–6617 (2006). https://doi.org/10.1038/sj.onc.1209675
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209675