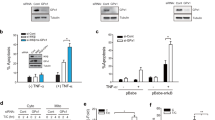
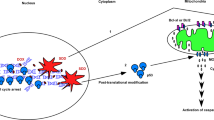
Abstract
Proline oxidase (POX), often considered a ‘housekeeping enzyme’ might play an important role in apoptosis. We have shown that POX generated proline-dependent reactive oxygen species (ROS), specifically superoxide radicals, and induced apoptosis through the mitochondrial (intrinsic) pathway. In our current report, we used DLD-1 colorectal cancer cells stably transfected with the POX gene under the control of a tetracycline-inducible promoter and found POX-stimulated expression of tumor necrosis factor-related apoptosis inducing ligand (TRAIL), DR5 and cleavage of caspase-8. Importantly, apoptosis measured by flow cytometry was partially inhibited by Z-IETD-FMK, a specific inhibitor of caspase-8. These findings suggest that the extrinsic (death receptor) pathway also is activated by POX. Furthermore, the mechanism of this effect on the extrinsic pathway, specifically, the induction of TRAIL by POX, may be mediated by NFAT transcription factors. Additionally, POX expression also dramatically decreased phosphorylation of MEK and ERK, and the decrease was partially reversed by expression of manganese superoxide dismutase (MnSOD). Overexpression of constitutively active form of MEK, acMEK, partially blocked POX-induced apoptosis. These findings suggest the involvement of MEK/ERK signaling and further confirm the role of ROS/superoxides in POX-induced apoptosis. Combined with previously published data, we conclude that POX may induce apoptosis through both intrinsic and extrinsic pathways and is involved in nuclear factor of activated T cells (NFAT) signaling and regulation of the MEK/ERK pathway. It is suggested that, as a nutrition factor, POX may modulate apoptosis signals induced by p53 or other anti-cancer agents and enhance apoptosis in stress situations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Abbreviations
- Ad:
-
adenovirus
- Dox:
-
doxycycline
- DR5:
-
death receptor 5
- MnSOD:
-
manganese superoxide dismutase
- MOI:
-
multiplicity of infection
- NFAT:
-
nuclear factor of activated T cells
- P5C:
-
pyrroline-5-carboxylate
- PBS:
-
phosphate-buffered saline
- PIG:
-
p53-induced gene
- POX:
-
proline oxidase
- ROS:
-
reactive oxygen species
- TRAIL:
-
tumor necrosis factor-related apoptosis inducing ligand
References
Adams E . (1970). Int Rev Connect Tissue Res 5: 1–91.
Cai J, Jones DP . (1998). J Biol Chem 273: 11401–11404.
Crabtree GR, Olson EN . (2002). Cell 109(Suppl): S67–79.
Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM . (2000). Exp Cell Res 256: 34–41.
Delwing D, Bavaresco CS, Chiarani F, Wannmacher CM, Wajner M, Dutra-Filho CS et al. (2003). Brain Res 991: 180–186.
Donald SP, Sun XY, Hu CA, Yu J, Mei JM, Valle D et al. (2001). Cancer Res 61: 1810–1815.
Duque J, Fresno M, Iniguez MA . (2005). J Biol Chem 280: 8686–8693.
Dussmann H, Kogel D, Rehm M, Prehn JH . (2003). J Biol Chem 278: 12645–12649.
Hagedorn CH, Phang JM . (1983). Arch Biochem Biophys 225: 95–101.
Hamilton G, Olszewski U, Ulsperger E, Baumgartner G . (2004). J Clin Oncol 22: 3164.
Holmstrom TH, Schmitz I, Soderstrom TS, Poukkula M, Johnson VL, Chow SC et al. (2000). EMBO J 19: 5418–5428.
Kelley SK, Ashkenazi A . (2004). Curr Opin Pharmacol 4: 333–339.
Latinis KM, Norian LA, Eliason SL, Koretzky GA . (1997). J Biol Chem 272: 31427–31434.
Liu Y, Borchert GL, Donald SP, Surazynski A, Hu CA, Weydert CJ et al. (2005). Carcinogenesis 26: 1335–1342.
Loo G . (2003). J Nutr Biochem 14: 64–73.
Lu KP . (2003). Cancer Cell 4: 175–180.
Macias MJ, Wiesner S, Sudol M . (2002). FEBS Lett 513: 30–37.
Maxwell SA, Rivera A . (2003). J Biol Chem 278: 9784–9789.
McCaffrey PG, Goldfeld AE, Rao A . (1994). J Biol Chem 269: 30445–30450.
Nakano K, Vousden KH . (2001). Mol Cell 7: 683–694.
Pani G, Colavitti R, Bedogni B, Fusco S, Ferraro D, Borrello S . (2004). Curr Med Chem 11: 1299–1308.
Phang JM . (1985). Curr Top Cell Regul 25: 91–132.
Phang JM, Downing SJ, Yeh GC, Smith RJ, Williams JA, Hagedorn CH et al. (1982). J Cell Physiol 110: 255–261.
Phang JM, Hu C-A, Valle D . (2001). The Metabolic and Molecular Bases of Inherited Disease. Scriver CR, Beaudet AL, Sly WS and Valle D (eds). McGraw Hill: New York, pp 1821–1838.
Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B . (1997). Nature 389: 300–305.
Rivera A, Maxwell SA . (2005). J Biol Chem 280: 29346–29354.
Semenza GL . (2003). Nat Rev Cancer 3: 721–732.
Soderstrom TS, Poukkula M, Holmstrom TH, Heiskanen KM, Eriksson JE . (2002). J Immunol 169: 2851–2860.
Tran SE, Holmstrom TH, Ahonen M, Kahari VM, Eriksson JE . (2001). J Biol Chem 276: 16484–16490.
Wada T, Penninger JM . (2004). Oncogene 23: 2838–2849.
Wang Q, Ji Y, Wang X, Evers BM . (2000). Biochem Biophys Res Commun 276: 466–471.
Wenzel U, Kuntz S, De Sousa UJ, Daniel H . (2003). Int J Cancer 106: 666–675.
Wenzel U, Nickel A, Kuntz S, Daniel H . (2004). Carcinogenesis 25: 703–712.
Wilson DJ, Alessandrini A, Budd RC . (1999). Cell Immunol 194: 67–77.
Wu GS, Burns TF, McDonald III ER, Jiang W, Meng R, Krantz ID et al. (1997). Nat Genet 17: 141–143.
Wu GS, Kim K, el-Deiry WS . (2000). Adv Exp Med Biol 465: 143–151.
Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L . (2003). Proc Natl Acad Sci USA 100: 1931–1936.
Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B . (2001). Mol Cell 7: 673–682.
Acknowledgements
This research is supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This project also has been funded in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. NO1-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, Y., Borchert, G., Surazynski, A. et al. Proline oxidase activates both intrinsic and extrinsic pathways for apoptosis: the role of ROS/superoxides, NFAT and MEK/ERK signaling. Oncogene 25, 5640–5647 (2006). https://doi.org/10.1038/sj.onc.1209564
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209564
Keywords
This article is cited by
-
Proline dehydrogenase in cancer: apoptosis, autophagy, nutrient dependency and cancer therapy
Amino Acids (2021)
-
Proline metabolism and redox; maintaining a balance in health and disease
Amino Acids (2021)
-
Proline metabolism in cancer
Amino Acids (2021)
-
The Janus-like role of proline metabolism in cancer
Cell Death Discovery (2020)
-
Understanding the role of key amino acids in regulation of proline dehydrogenase/proline oxidase (prodh/pox)-dependent apoptosis/autophagy as an approach to targeted cancer therapy
Molecular and Cellular Biochemistry (2020)