Abstract
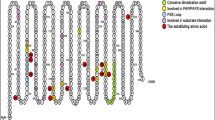
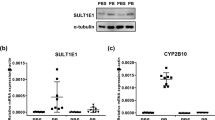
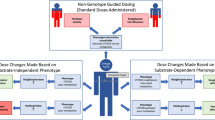
Cytosolic sulfotransferases (SULTs) are phase II detoxification enzymes that are involved in the biotransformation of a wide variety of structurally diverse endo- and xenobiotics, including many therapeutic agents and endogenous steroids. Single-nucleotide polymorphisms (SNPs) in SULTs have functional consequences on the translated protein. For the most part, these SNPs are fairly uncommon in the population, but some, most notably for SULT isoform 1A1, are commonly found and have been associated with cancer risk for a variety of tumor sites and also with response to therapeutic agents. SNPs in the hydroxysteroid sulfotransferase, SULT2A1, have been identified in African-American subjects and influence the ratio of plasma DHEA:DHEA-S. This modification could potentially influence cancer risk in steroidogenic tissues. SNPs in many SULTs are ethnically distributed, another factor that could influence SULT pharmacogenetics. Finally, genetic variation has also been identified in 3′-phosphoadenoside 5′-phosphosulfate synthetase (PAPPS), the enzymes responsible for producing the obligatory cosubstrate for all sulfotransferases. Taken together, this variability could substantially influence the disposition of drugs metabolized by SULTs. Elucidation of the basis and effect of variability in sulfation could greatly impact individualized therapy in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Consensus Conference. (1985). JAMA 254: 3461–3463.
Consensus Conference. (1986). CA Cancer J Clin 36: 42–47.
Anderson RJ, Jackson BL, Liebentritt DK . (1988). J Lab Clin Med 112: 773–783.
Arlt W, Haas J, Callies F, Reincke M, Hubler D, Oettel M et al. (1999). J Clin Endocrinol Metab 84: 2170–2176.
Besset S, Vincourt JB, Amalric F, Girard JP . (2000). FASEB J 14: 345–354.
Brittelli A, De Santi C, Raunio H, Pelkonen O, Rossi G, Pacifici GM . (1999). Eur J Clin Pharmacol 55: 691–695.
Campbell NR, Van Loon JA, Weinshilboum RM . (1987). Biochem Pharmacol 36: 1435–1446.
Cappiello M, Franchi M, Giuliani L, Pacifici GM . (1989). Eur J Clin Pharmacol 37: 317–320.
Duanmu Z, Kocarek TA, Runge-Morris M . (2001). Drug Metab Dispos 29: 1130–1135.
Falany CN, Comer KA, Dooley TP, Glatt H . (1995). Ann NY Acad Sci 774: 59–72.
Falany CN, Wheeler J, Oh TS, Falany JL . (1994a). J Steroid Biochem Mol Biol 48: 369–375.
Falany CN, Wheeler J, Oh TS, Falany JL . (1994b). J Steroid Biochem Mol Biol 48: 369–375.
Falany JL, Azziz R, Falany CN . (1998). Chem Biol Interact 109: 329–339.
Falany JL, Falany CN . (1996). Cancer Res 56: 1551–1555.
Fang HL, Shenoy S, Duanmu Z, Kocarek TA, Runge-Morris M . (2003). Drug Metab Dispos 31: 1378–1381.
Glatt H . (2000). Chem Biol Interact 129: 141–170.
Glatt H, Meinl W . (2004). Naunyn Schmiedebergs Arch Pharmacol 369: 55–68.
Hashimoto Y, Hirschberg CB . (1992). Tanpakushitsu Kakusan Koso 37: 1701–1706.
Hempel N, Wang H, LeCluyse EL, McManus ME, Negishi M . (2004). Mol Pharmacol 66: 1690–1701.
Hume R, Barker EV, Coughtrie MW . (1996). Histochem Cell Biol 105: 147–152.
Huttner WB . (1988). Annu Rev Physiol 50: 363–376.
Kester MH, Kaptein E, Roest TJ, van Dijk CH, Tibboel D, Meinl W et al. (1999). J Clin Endocrinol Metab 84: 1357–1364.
Khetawat G, Faraday N, Nealen ML, Vijayan KV, Bolton E, Noga SJ et al. (2000). Blood 95: 2289–2296.
Kotov A, Falany JL, Wang J, Falany CN . (1999). J Steroid Biochem Mol Biol 68: 137–144.
Kudlacek PE, Clemens DL, Anderson RJ . (1995). Biochem Biophys Res Commun 210: 363–369.
Li X, Clemens DL, Cole JR, Anderson RJ . (2001). J Endocrinol 171: 525–532.
Liyou NE, Buller KM, Tresillian MJ, Elvin CM, Scott HL, Dodd PR et al. (2003). J Histochem Cytochem 51: 1655–1664.
Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC et al. (1992). N Engl J Med 326: 852–856.
Marazziti D, Palego L, Mazzanti C, Silvestri S, Cassano GB . (1995). Chronobiol Int 12: 100–105.
Marazziti D, Palego L, Rossi A, Cassano GB . (1998). Neuropsychobiology 38: 1–5.
Mariotto A, Capocaccia R, Verdecchia A, Micheli A, Feuer EJ, Pickle L et al. (2002). Cancer Causes Control 13: 101–111.
Mortola JF, Yen SS . (1990). J Clin Endocrinol Metab 71: 696–704.
Nagata K, Yamazoe Y . (2000). Annu Rev Pharmacol Toxicol 40: 159–176.
Ning B, Nowell S, Sweeney C, Ambrosone CB, Williams S, Miao X et al. (2005). Pharmacogenet Genomics 15: 465–473.
Nishiyama T, Ogura K, Nakano H, Ohnuma T, Kaku T, Hiratsuka A et al. (2002). Biochem Pharmacol 63: 1817–1830.
Nowell S, Ambrosone CB, Ozawa S, MacLeod SL, Mrackova G, Williams S et al. (2000). Pharmacogenetics 10: 789–797.
Nowell S, Sweeney C, Winters M, Stone A, Lang NP, Hutchins LF et al. (2002). J Natl Cancer Inst 94: 1635–1640.
Ozawa S, Tang YM, Yamazoe Y, Kato R, Lang NP, Kadlubar FF . (1998). Chem Biol Interact 109: 237–248.
Parker Jr CR . (1999). Steroids 64: 640–647.
Raftogianis RB, Wood TC, Otterness DM, Van Loon JA, Weinshilboum RM . (1997). Biochem Biophys Res Commun 239: 298–304.
Spink BC, Katz BH, Hussain MM, Pang S, Connor SP, Aldous KM et al. (2000). Carcinogenesis 21: 1947–1957.
Sundaram RS, Szumlanski C, Otterness D, van Loon JA, Weinshilboum RM . (1989a). Drug Metab Dispos 17: 255–264.
Sundaram RS, Van Loon JA, Tucker R, Weinshilboum RM . (1989b). Clin Pharmacol Ther 46: 501–509.
Tarantino MD, Kunicki TJ, Nugent DJ . (1994). Ann NY Acad Sci 714: 293–296.
Thomae BA, Eckloff BW, Freimuth RR, Wieben ED, Weinshilboum RM . (2002). Pharmacogenomics J 2: 48–56.
Van Loon J, Weinshilboum RM . (1984). Biochem Genet 22: 997–1014.
Visser TJ, Kaptein E, Glatt H, Bartsch I, Hagen M, Coughtrie MW . (1998). Chem Biol Interact 109: 279–291.
Wegman P, Vainikka L, Stal O, Nordenskjold B, Skoog L, Rutqvist LE et al. (2005). Breast Cancer Res 7: R284–R290.
Weinshilboum R . (1990). Pharmacol Ther 45: 93–107.
Weinshilboum R, Aksoy I . (1994). Chem Biol Interact 92: 233–246.
Wilborn T, Lang N, Smith M, Meleth S, Falany C . (2005). J Steroid Biochem Mol Biol, in press.
Xu ZH, Freimuth RR, Eckloff B, Wieben E, Weinshilboum RM . (2002). Pharmacogenetics 12: 11–21.
Xu ZH, Otterness DM, Freimuth RR, Carlini EJ, Wood TC, Mitchell S et al. (2000). Biochem Biophys Res Commun 268: 437–444.
Xu ZH, Thomae BA, Eckloff BW, Wieben ED, Weinshilboum RM . (2003). Biochem Pharmacol 65: 1787–1796.
Young Jr WF, Laws Jr ER, Sharbrough FW, Weinshilboum RM . (1985). J Neurochem 44: 1131–1137.
Zou JY, Petney R, Roth JA . (1990). J Neurochem 55: 1154–1158.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Nowell, S., Falany, C. Pharmacogenetics of human cytosolic sulfotransferases. Oncogene 25, 1673–1678 (2006). https://doi.org/10.1038/sj.onc.1209376
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209376