Abstract
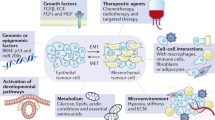
Epithelial–mesenchymal transition (EMT) is an important process during development by which epithelial cells acquire mesenchymal, fibroblast-like properties and show reduced intercellular adhesion and increased motility. Accumulating evidence points to a critical role of EMT-like events during tumor progression and malignant transformation, endowing the incipient cancer cell with invasive and metastatic properties. Several oncogenic pathways (peptide growth factors, Src, Ras, Ets, integrin, Wnt/β-catenin and Notch) induce EMT and a critical molecular event is the downregulation of the cell adhesion molecule E-cadherin. Recently, activation of the phosphatidylinositol 3′ kinase (PI3K)/AKT axis is emerging as a central feature of EMT. In this review, we discuss the role of PI3K/AKT pathways in EMT during development and cancer with a focus on E-cadherin regulation. Interactions between PI3K/AKT and other EMT-inducing pathways are presented, along with a discussion of the therapeutic implications of modulating EMT in order to achieve cancer control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Altomare DA and Testa JR . (2005). Oncogene Rev., 24, 7455–7464.
Amiel J and Lyonnet S . (2001). J. Med. Genet., 38, 729–739.
Bachelder RE, Yoon SO, Franci C, de Herreros AG and Mercurio AM . (2005). J. Cell Biol., 168, 29–33.
Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL and Arteaga CL . (2000). J. Biol. Chem., 275, 36803–36810.
Barbera MJ, Puig I, Dominguez D, Julien-Grille S, Guaita-Esteruelas S, Peiro S, Baulida J, Franci C, Dedhar S, Larue L and Garcia de Herreros A . (2004). Oncogene, 23, 7345–7354.
Barbieri MA, Kohn AD, Roth RA and Stahl PD . (1998). J. Biol. Chem., 273, 19367–19370.
Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J and Garcia De Herreros A . (2000). Nat. Cell Biol., 2, 84–89.
Bellacosa A, de Feo D, Godwin AK, Bell DW, Cheng JQ, Altomare DA, Wan M, Dubeau L, Scambia G, Masciullo V, Ferrandina G, Panici PB, Mancuso S, Neri G and Testa JR . (1995). Int. J. Cancer, 64, 280–285.
Bellacosa A, Kumar CC, Di Cristofano A and Testa JR . (2005). Adv. Cancer Res., 94, 29–86.
Bellacosa A, Testa JR, Moore R and Larue L . (2004). Cancer Biol. Ther., 3, 268–275.
Bellacosa A, Testa JR, Staal SP and Tsichlis PN . (1991). Science, 254, 274–277.
Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL and Moses HL . (2001). Mol. Biol. Cell, 12, 27–36.
Birchmeier W and Behrens J . (1994). Biochim. Biophys. Acta, 1198, 11–26.
Boyer B, Roche S, Denoyelle M and Thiery JP . (1997). EMBO J., 16, 5904–5913.
Boyer B, Valles AM and Edme N . (2000). Biochem. Pharmacol., 60, 1091–1099.
Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A and Kirchner T . (2005). Cells Tissues Organs, 179, 56–65.
Brognard J, Clark AS, Ni Y and Dennis PA . (2001). Cancer Res., 61, 3986–3997.
Butz S and Larue L . (1995). Cell Adhes. Commun., 3, 337–352.
Cacheux V, Dastot-Le Moal F, Kaariainen H, Bondurand N, Rintala R, Boissier B, Wilson M, Mowat D and Goossens M . (2001). Hum. Mol. Genet., 10, 1503–1510.
Camenisch TD, Schroeder JA, Bradley J, Klewer SE and McDonald JA . (2002). Nat. Med., 8, 850–855.
Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA . (2000). Nat. Cell Biol., 2, 76–83.
Chambers AF, Groom AC and MacDonald IC . (2002). Nat. Rev. Cancer, 2, 563–572.
Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC, Tsichlis PN and Testa JR . (1992). Proc. Natl. Acad. Sci. USA, 89, 9267–9271.
Cheng JQ, Lindsley CW, Cheng GZ, Yang H and Nicosia SV . (2005). Oncogene Rev., 24, 7482–7492.
Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK and Testa JR . (1996). Proc. Natl. Acad. Sci. USA, 93, 3636–3641.
Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P and Ben-Ze'ev A . (2003). J. Cell Biol., 163, 847–857.
De Craene B, Gilbert B, Stove C, Bruyneel E, van Roy F and Berx G . (2005). Cancer Res., 65, 6237–6244.
Delcommenne M, Tan C, Gray V, Rue L, Woodgett J and Dedhar S . (1998). Proc. Natl. Acad. Sci. USA, 95, 11211–11216.
Edme N, Downward J, Thiery JP and Boyer B . (2002). J. Cell Sci., 115, 2591–2601.
Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, Perrella MA and Lee ME . (2001). J. Biol. Chem., 276, 17479–17483.
Gavrilovic J, Moens G, Thiery JP and Jouanneau J . (1990). Cell Regul., 1, 1003–1014.
Gilles C, Polette M, Birembaut P, Brunner N and Thompson EW . (1997). Clin. Exp. Metast., 15, 519–526.
Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN and Larue L . (2003). Cancer Res., 63, 2172–2178.
Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A and Reeve AE . (1998). Nature, 392, 402–405.
Hsu T, Trojanowska M and Watson DK . (2004). J. Cell. Biochem., 91, 896–903.
Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, Van Eeden F, Cuppen E, Zivkovic D, Plasterk RH and Clevers H . (2003). Nature, 425, 633–637.
Kang Y and Massague J . (2004). Cell, 118, 277–279.
Kemler R, Hierholzer A, Kanzler B, Kuppig S, Hansen K, Taketo MM, de Vries WN, Knowles BB and Solter D . (2004). Development, 131, 5817–5824.
Kim D, Kim S, Koh H, Yoon SO, Chung AS, Cho KS and Chung J . (2001). FASEB J., 15, 1953–1962.
Krymskaya VP, Hoffman R, Eszterhas A, Ciocca V and Panettieri Jr RA . (1997). Am. J. Physiol., 273, L1220–1227.
Kumar CC and Madison V . (2005). Oncogene Rev., 24, 7493–7501.
Larue L, Antos C, Butz S, Huber O, Delmas V, Dominis M and Kemler R . (1996). Development, 122, 3185–3194.
Larue L, Ohsugi M, Hirchenhain J and Kemler R . (1994). Proc. Natl. Acad. Sci. USA, 91, 8263–8267.
Lee YI, Kwon YJ and Joo CK . (2004). Biochem. Biophys. Res. Commun., 316, 997–1001.
Leptin M and Grunewald B . (1990). Development, 110, 73–84.
Logan CY, Miller JR, Ferkowicz MJ and McClay DR . (1999). Development, 126, 345–357.
Majumder PK and Sellers WR . (2005). Oncogene Rev., 24, 7465–7474.
Morali OG, Delmas V, Moore R, Jeanney C, Thiery JP and Larue L . (2001). Oncogene, 20, 4942–4950.
Morali OG, Jouneau A, McLaughlin KJ, Thiery JP and Larue L . (2000). Dev. Biol., 227, 133–145.
Muraoka-Cook RS, Dumont N and Arteaga CL . (2005). Clin. Cancer Res., 11, 937s–943s.
Murillo MM, del Castillo G, Sanchez A, Fernandez M and Fabregat I . (2005). Oncogene, 24, 4580–4587.
Novak A, Hsu SC, Leung-Hagesteijn C, Radeva G, Papkoff J, Montesano R, Roskelley C, Grosschedl R and Dedhar S . (1998). Proc. Natl. Acad. Sci. USA, 95, 4374–4379.
Palmer HG, Larriba MJ, Garcia JM, Ordonez-Moran P, Pena C, Peiro S, Puig I, Rodriguez R, de la Fuente R, Bernad A, Pollan M, Bonilla F, Gamallo C, de Herreros AG and Munoz A . (2004). Nat. Med., 10, 917–919.
Park BK, Zeng X and Glazer RI . (2001). Cancer Res., 61, 7647–7653.
Perkins J, St John J and Ahmed A . (2002). Mol. Med., 8, 847–856.
Piek E, Moustakas A, Kurisaki A, Heldin CH and ten Dijke P . (1999). J. Cell Sci., 112, 4557–4568.
Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G and Comoglio PM . (1994). Cell, 77, 261–271.
Qiu Q, Yang M, Tsang BK and Gruslin A . (2004). Reproduction, 128, 355–363.
Quilliam LA, Castro AF, Rogers-Graham KS, Martin CB, Der CJ and Bi C . (1999). J. Biol. Chem., 274, 23850–23857.
Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z and Bissell MJ . (2005). Nature, 436, 123–127.
Ringel MD, Hayre N, Saito J, Saunier B, Schuppert F, Burch H, Bernet V, Burman KD, Kohn LD and Saji M . (2001). Cancer Res., 61, 6105–6111.
Rodrigo I, Cato AC and Cano A . (1999). Exp. Cell Res., 248, 358–371.
Salomon DS, Bianco C, Ebert AD, Khan NI, De Santis M, Normanno N, Wechselberger C, Seno M, Williams K, Sanicola M, Foley S, Gullick WJ and Persico G . (2000). Endocr. Relat. Cancer, 7, 199–226.
Samuels Y, Diaz Jr LA, Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, Rago C, Huso DL, Lengauer C, Kinzler KW, Vogelstein B and Velculescu VE . (2005). Cancer Cell, 7, 561–573.
Samuels Y and Velculescu VE . (2004). Cell Cycle, 3, 1221–1224.
Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B and Velculescu VE . (2004). Science, 304, 554.
Shepherd T and Hassell JA . (2001). J. Mammary Gland Biol. Neoplasia, 6, 129–140.
Soo K, O'Rourke MP, Khoo PL, Steiner KA, Wong N, Behringer RR and Tam PP . (2002). Dev. Biol., 247, 251–270.
Staal SP . (1987). Proc. Natl. Acad. Sci. USA, 84, 5034–5037.
Strizzi L, Bianco C, Normanno N, Seno M, Wechselberger C, Wallace-Jones B, Khan NI, Hirota M, Sun Y, Sanicola M and Salomon DS . (2004). J. Cell. Physiol., 201, 266–276.
Sun M, Wang G, Paciga JE, Feldman RI, Yuan ZQ, Ma XL, Shelley SA, Jove R, Tsichlis PN, Nicosia SV and Cheng JQ . (2001). Am. J. Pathol., 159, 431–437.
Suriano G, Oliveira C, Ferreira P, Machado JC, Bordin MC, De Wever O, Bruyneel EA, Moguilevsky N, Grehan N, Porter TR, Richards FM, Hruban RH, Roviello F, Huntsman D, Mareel M, Carneiro F, Caldas C and Seruca R . (2003). Hum. Mol. Genet., 12, 575–582.
Taki M, Kamata N, Yokoyama K, Fujimoto R, Tsutsumi S and Nagayama M . (2003). Cancer Sci., 94, 593–597.
Tan C, Costello P, Sanghera J, Dominguez D, Baulida J, de Herreros AG and Dedhar S . (2001). Oncogene, 20, 133–140.
Testa JR and Bellacosa A . (2001). Proc. Natl. Acad. Sci. USA, 98, 10983–10985.
Testa JR and Tsichlis PN . (2005). Oncogene Rev., 24, 7391–7393.
Thiery JP . (2002). Nat. Rev. Cancer, 2, 442–454.
Thiery JP . (2003). Curr. Opin. Genet. Dev., 13, 365–371.
Thiery JP and Morgan M . (2004). Nat. Med., 10, 777–778.
Thisse B, el Messal M and Perrin-Schmitt F . (1987). Nucleic Acids Res., 15, 3439–3453.
Tian Q, Feetham MC, Tao WA, He XC, Li L, Aebersold R and Hood L . (2004). Proc. Natl. Acad. Sci. USA, 101, 15370–15375.
Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC and de la Pompa JL . (2004). Genes Dev., 18, 99–115.
Valles AM, Boyer B, Badet J, Tucker GC, Barritault D and Thiery JP . (1990). Proc. Natl. Acad. Sci. USA, 87, 1124–1128.
Van de Putte T, Maruhashi M, Francis A, Nelles L, Kondoh H, Huylebroeck D and Higashi Y . (2003). Am. J. Hum. Genet., 72, 465–470.
Veeraraghavalu K, Subbaiah VK, Srivastava S, Chakrabarti O, Syal R and Krishna S . (2005). J. Virol., 79, 7889–7898.
Vestweber D, Gossler A, Boller K and Kemler R . (1987). Dev. Biol., 124, 451–456.
Vestweber D and Kemler R . (1984). Exp. Cell Res., 152, 169–178.
Vleminckx K, Vakaet Jr L, Mareel M, Fiers W and van Roy F . (1991). Cell, 66, 107–119.
Wakamatsu N, Yamada Y, Yamada K, Ono T, Nomura N, Taniguchi H, Kitoh H, Mutoh N, Yamanaka T, Mushiake K, Kato K, Sonta S and Nagaya M . (2001). Nat. Genet., 27, 369–370.
Ward KR, Zhang KX, Somasiri AM, Roskelley CD and Schrader JW . (2004). Oncogene, 23, 1187–1196.
Watanabe K and Yamaguchi Y . (1996). J. Biol. Chem., 271, 22945–22948.
Weinstein IB . (2002). Science, 297, 63–64.
Weston CR and Davis RJ . (2001). Science, 292, 2439–2440.
Wu C, Keightley SY, Leung-Hagesteijn C, Radeva G, Coppolino M, Goicoechea S, McDonald JA and Dedhar S . (1998). J. Biol. Chem., 273, 528–536.
Wu C . (1999). J. Cell Sci., 112 (Part 24), 4485–4489.
Yamada K, Yamada Y, Nomura N, Miura K, Wakako R, Hayakawa C, Matsumoto A, Kumagai T, Yoshimura I, Miyazaki S, Kato K, Sonta S, Ono H, Yamanaka T, Nagaya M and Wakamatsu N . (2001). Am. J. Hum. Genet., 69, 1178–1185.
Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and Weinberg RA . (2004). Cell, 117, 927–939.
Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S and Kumar R . (2005). Cancer Res., 65, 3179–3184.
Yook JI, Li XY, Ota I, Fearon ER and Weiss SJ . (2005). J. Biol. Chem., 280, 11740–11748.
Yuan ZQ, Sun M, Feldman RI, Wang G, Ma X, Jiang C, Coppola D, Nicosia SV and Cheng JQ . (2000). Oncogene, 19, 2324–2330.
Zoltan-Jones A, Huang L, Ghatak S and Toole BP . (2003). J. Biol. Chem., 278, 45801–45810.
Acknowledgements
This work was supported by grants from the Ligue Nationale Contre le Cancer (Equipe labellisée) and Cancéropole, NIH Grants CA105008, CA06927, and an appropriation from the Commonwealth of Pennsylvania to the Fox Chase Cancer Center. We would like to thank Drs Brigitte Boyer, John Burch, Jonathan Chernoff and Beatrice Mintz for helpful discussion and critical reading of the manuscript; all the members of their laboratories for helpful discussions; and Rose Sonlin for expert secretarial assistance.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Larue, L., Bellacosa, A. Epithelial–mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 24, 7443–7454 (2005). https://doi.org/10.1038/sj.onc.1209091
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209091
Keywords
This article is cited by
-
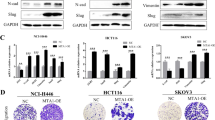
K-Ras(V12) differentially affects the three Akt isoforms in lung and pancreatic carcinoma cells and upregulates E-cadherin and NCAM via Akt3
Cell Communication and Signaling (2024)
-
ADAMTS18 deficiency associates extracellular matrix dysfunction with a higher risk of HER2-positive mammary tumorigenesis and metastasis
Breast Cancer Research (2024)
-
PLAC8 contributes to the malignant behaviors of cervical cancer cells by activating the SOX4-mediated AKT pathway
Histochemistry and Cell Biology (2023)
-
DNA methyltransferase 1 knockdown reverses PTEN and VDR by mediating demethylation of promoter and protects against renal injuries in hepatitis B virus-associated glomerulonephritis
Cell & Bioscience (2022)
-
Acacetin inhibits invasion, migration and TGF-β1-induced EMT of gastric cancer cells through the PI3K/Akt/Snail pathway
BMC Complementary Medicine and Therapies (2022)