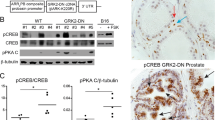
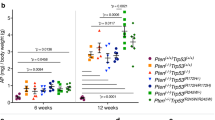
Abstract
Circulating insulin-like growth factor-I (IGF-I) levels have been shown to be related to risk of prostate cancer in epidemiologic studies. While specific genetic loci responsible for interindividual variation in circulating IGF-I levels in normal men have not been identified, candidate genes include those involved in the growth hormone (GH)–IGF-I axis such as the hypothalamic factors GH releasing hormone (GHRH) and somatostatin and their receptors. To investigate the role of the GH–IGF-I axis on in vivo prostate carcinogenesis and neoplastic progression, we generated mice genetically predisposed to prostate cancer (the TRAMP model) to be homozygous for lit, a mutation that inactivates the GHRH receptor (GHRH-R) and reduces circulating levels of GH and IGF-I. The lit mutation significantly reduced the percentage of the prostate gland showing neoplastic changes at 35 weeks of age (P=0.0005) and was also associated with improved survival (P<0.01). These data provide an example of a germ line mutation that reduces risk in an experimental prostate carcinogenesis model. The results suggest that prostate carcinogenesis and progression may be influenced by germ line variation of genes encoding signalling molecules in the GH–IGF-I axis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Abbreviations
- IGF-I:
-
insulin-like growth factor I
- GH:
-
growth hormone
- GHRH-R:
-
growth hormone releasing hormone receptor
- IGFBP-3:
-
insulin-like growth factor binding protein-3
- TRAMP:
-
transgenic adenocarcinoma of the mouse prostate
- PIN:
-
prostatic intraepithelial neoplasia
- nt:
-
nucleotide
References
Aleppo G, Moskal II SF, De Grandis PA, Kineman RD and Frohman LA . (1997). Endocrinology, 138, 1058–1065.
Arantes-Oliveira N, Berman JR and Kenyon C . (2003). Science, 302, 611.
Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH and Pollak M . (1998). Science, 279, 563–566.
Chandrashekar V, Zaczek D and Bartke A . (2004). Biol. Reprod., 71, 17–27.
Chopin LK and Herington AC . (2001). Prostate, 49, 116–121.
Cohen P, Peehl DM, Lamson G and Rosenfeld RG . (1991). J. Clin. Endocrinol. Metab., 73, 401–407.
Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G and Klocker H . (1994). Cancer Res., 54, 5474–5478.
Dillin A, Crawford DK and Kenyon C . (2002). Science, 298, 830–834.
Flurkey K, Papaconstantinou J, Miller RA and Harrison DE . (2001). Proc. Natl. Acad. Sci. USA, 98, 6736–6741.
Gingrich JR, Barrios RJ, Foster BA and Greenberg NM . (1999). Prostate Cancer Prostatic Dis., 2, 70–75.
Godfrey P, Rahal JO, Beamer WG, Copeland NG, Jenkins NA and Mayo KE . (1993). Nat. Genet., 4, 227–232.
Greenberg NM, DeMayo FJ, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ and Rosen JM . (1995). Proc. Natl. Acad. Sci. USA, 92, 3439–3443.
Guarente L and Kenyon C . (2000). Nature, 408, 255–262.
Harrela M, Koinstinen H, Kaprio J, Lehtovirta M, Tuomilehto J, Eriksson J, Toivanen L, Koskenvuo M, Leinonen P, Koistinene R and Seppala M . (1996). J. Clin. Invest., 98, 2612–2615.
Hellawell GO, Turner GD, Davies DR, Poulsom R, Brewster SF and Macaulay VM . (2002). Cancer Res., 62, 2942–2950.
Holzenberger M, Dupont J, Ducos B, Leneuve P, Geleon A, Even PC, Cervera P and Le Bouc Y . (2002). Nature, 421, 125–126.
Jansson JO, Downs TR, Beamer WG and Frohman LA . (1986). Science, 232, 511–512.
Kaplan-Lefko PJ, Chen T-M, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, Maddison LA, Foster BA and Greenberg NM . (2003). Prostate, 55, 219–237.
Kenyon C . (2001). Cell, 105, 165–168.
Letsch M, Schally AV, Busto R, Bajo AM and Varga JL . (2003). Proc. Natl. Acad. Sci. USA, 100, 1250–1255.
Lin SC, Lin CR, Gukovsky I, Lusis AJ, Sawchenko PE and Rosenfeld MG . (1993). Nature, 364, 208–213.
Longo VD and Finch CE . (2003). Science, 299, 1342–1346.
Maheshwari HG, Silverman BL, Dupuis J and Baumann G . (1998). J. Clin. Endocrinol. Metab., 83, 4065–4074.
O'Connor R, Fennelly C and Krausse D . (2000). Biochem. Soc. Trans., 28, 47–51.
Peng XD, Park S, Gadelha MR, Coschigano KT, Kopchick JJ, Frohman LA and Kineman RD . (2001). Endocrinology, 142, 1117–1123.
Pollak M, Beamer W and Zhang JC . (1999). Cancer Metastasis Rev., 17, 383–390.
Pollak M, Schernhammer ES and Hankinson SE . (2004). Nat. Rev. Cancer, 4, 505–518.
Pollak M . (2001). Epidemiol. Rev., 23, 59–66.
Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM and Egger M . (2004). Lancet, 363, 1346–1353.
Shaneyfelt T, Husein R, Bubley GJ and Mantzoros CS . (2000). J. Clin. Oncol., 18, 847–853.
Stattin P, Rinaldi S, Biessy C, Stenman UH, Hallmans G and Kaaks R . (2004). J. Clin. Oncol., 22, 3104–3112.
Tatar M, Bartke A and Antebi A . (2003). Science, 299, 1346–1351.
Tissenbaum HA and Guarente L . (2002). Dev. Cell, 1, 9–19.
Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Hee Park S, Thompson T, Karsenty G, Bradley A and Donehower LA . (2002). Nature, 415, 45–53.
Wajnrajch MP, Gertner JM, Harbison MD, Chua SC and Leibel RL . (1996). Nat. Genet., 12, 88–90.
Wang J, Eltoum IE and Lamartiniere CA . (2004). Mol. Cell Endocrinol., 219, 171–180.
Wolk A, Mantzoros CS, Andersson SO, Bergstrom R, Signorello LB, Lagiou P, Adami HO and Trichopoulos D . (1998). J. Natl. Cancer Inst., 90, 911–915.
Acknowledgements
We thank Dr Michael Ittmann for assistance with pathological examination of the mouse tissues, Julie Bédard for the work on GHRH-R mRNA detection, Kathy-Ann Forner, Scott Hartigan and Danielle Couture for technical assistance with the breeding program, and Martine Bourdeau for assistance in Ki-67 immunostains. This work was funded by CPCRI-IDEA to Dr M Pollak. P Gaudreau is recipient of a scholarship chercheur-boursier national from FRSQ.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Majeed, N., Blouin, MJ., Kaplan-Lefko, P. et al. A germ line mutation that delays prostate cancer progression and prolongs survival in a murine prostate cancer model. Oncogene 24, 4736–4740 (2005). https://doi.org/10.1038/sj.onc.1208572
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208572
Keywords
This article is cited by
-
Role of diet in prostate cancer: the epigenetic link
Oncogene (2015)
-
Modulation of insulin/IGFs pathways by sirtuin-7 inhibition in drug-induced chemoreistance
Diagnostic Pathology (2014)
-
Growth hormone-releasing hormone antagonists abolish the transactivation of human epidermal growth factor receptors in advanced prostate cancer models
Investigational New Drugs (2014)
-
The influence of growth hormone/insulin-like growth factor deficiency on prostatic dysplasia in pbARR2-Cre, PTEN knockout mice
Prostate Cancer and Prostatic Diseases (2013)
-
The GH/IGF-1 axis in ageing and longevity
Nature Reviews Endocrinology (2013)