Abstract
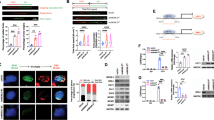
Siah-1 is the mammalian homolog of Drosophila seven in absentia (sina) and has been identified as a p53-inducible gene. Siah-1 can induce cell cycle arrests, tumor suppression, and apoptosis through a novel β-catenin degradation pathway. To determine whether genetic alterations of Siah-1 gene are involved in the development and/or progression of gastric cancer, we searched for mutation of the Siah-1 gene in 95 gastric cancers by single-strand conformational polymorphism and sequencing. The effect of Siah-1 on β-catenin degradation was further examined in wild- and mutant-type Siah-1-transfected HEK 293T cells. We found two missense mutations of the Siah-1 gene. The cases with Siah-1 mutation showed nuclear translocation and cytoplasmic staining of β-catenin. Interestingly, two mutants of Siah-1 stabilized cytoplasmic levels of β-catenin, even after treatment of adriamycin. Furthermore, both mutants failed to suppress cyclin D1 expression and to induce apoptosis. These data suggest that inactivating mutations of the Siah-1 may contribute to the development of gastric cancer through β-catenin stabilization and apoptosis block.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Amson RB, Nemani M, Roperch JP, Israeli D, Bougueleret L, Le Gall I, Medhioub M, Linares-Cruz G, Lethrosne F, Pasturaud P, Piouffre L, Prieur S, Susini L, Alvaro V, Millasseau P, Guidicelli C, Bui H, Massart C, Cazes L, Dufour F, Bruzzoni-Giovanelli H, Owadi H, Hennion C, Charpak G and Telerman A . (1996). Proc. Natl. Acad. Sci. USA, 93, 3953–3957.
Carthew RW and Rubin GM . (1990). Cell, 63, 561–577.
Clevers H and van de Wetering M . (1997). Trends Genet., 13, 485–489.
Della NG, Senior PV and Bowtell DL . (1993). Development, 117, 1333–1343.
Fiucci G, Beaucourt S, Duflaut D, Lespagnol A, Stumptner-Cuvelette P, Geant A, Buchwalter G, Tuynder M, Susini L, Lassalle J-M, Wasylyk C, Wasylyk B, Oren M, Amson R and Telerman A . (2004). Proc. Natl. Acad. Sci. USA, 101, 3510–3515.
Hsu SC, Galceran J and Grosschedl R . (1998). Mol. Cell. Biol., 18, 4807–4818.
Hu G, Chung YL, Glover T, Valentine V, Look AT and Fearon ER . (1997). Genomics, 46, 103–111.
Hu G and Fearon ER . (1999). Mol. Cell. Biol., 19, 724–732.
Kinzler KW and Vogelstein B . (1996). Cell, 87, 159–170.
Lee JY, Dong SM, Kim SY, Yoo NJ, Lee SH and Park WS . (1998). Virchows Arch., 433, 305–309.
Liu J, Stevens J, Rote CA, Yost HJ, Hu Y, Neufeld KK, White RL and Matsunami N . (2001). Mol. Cell, 7, 927–936.
Lustig B and Behrens J . (2003). J. Cancer Res. Clin. Oncol., 129, 199–221.
Matsuzawa S, Takayama S, Froesch BA, Zapata JM and Reed JC . (1998). EMBO J., 17, 2736–2747.
Matsuzawa S-I and Reed JC . (2001). Mol. Cell, 7, 915–926.
Nakatsuru S, Yanagisawa A, Ichii S, Tahara E, Kato Y, Nakamura Y and Horii A . (1992). Hum. Mol. Genet., 1, 559–563.
Nemani M, Linares-Cruz G, Bruzzoni-Giovanelli H, Roperch JP, Tuynder M, Bougueleret L, Cherif D, Medhioub M, Pasturaud P, Alvaro V, der Sarkissan H, Cazes L, Le Paslier D, Le Gall I, Israeli D, Dausset J, Sigaux F, Chumakov I, Oren M, Calvo F, Amson RB, Cohen D and Telerman A . (1996). Proc. Natl. Acad. Sci, USA, 93, 9039–9042.
Park WS, Oh RR, Kim YS, Park JY, Shin MS, Lee HK, Lee SH, Yoo NJ and Lee JY . (2000). APMIS, 108, 195–200.
Park WS, Oh RR, Park JY, Lee SH, Shin MS, Kim YS, Kim SY, Lee HK, Kim PJ, Oh ST, Yoo NJ and Lee JY . (1999). Cancer Res., 59, 4257–4260.
Peifer M and Polakis P . (2000). Science, 287, 1606–1609.
Polakis P . (2001). Cell, 105, 563–566.
Roperch JP, Lethrone F, Prieur S, Pioffre L, Israeli D, Tuynder M, Nemani M, Pasturaud P, Gendron MC, Dausset J, Oren M, Amson RB and Telerman A . (1999). Proc. Natl. Acad. Sci. USA, 96, 8070–8073.
Shtutman M, Zhurinsky J, Oren M, Levina E and Ben-Ze'ev A . (2002). Cancer Res., 62, 5947–5954.
Sivak MV and Jagelman DG . (1984). Gastrointest. Endosc., 30, 102–104.
Suh CI, Suh K-A, Park S-H, Chang HJ, Ko J-W and Ahn D-H . (2000). J. Korean Cancer Assoc., 32, 827–834.
Tetsu O and McCormick F . (1998). Nature, 398, 422–426.
Acknowledgements
We thank Dr Su-Jae Lee (Korea Institute of Radiological & Medical Sciences, Korea) for providing the p53 mutant expression vector system. This work was supported by the Korea Science & Engineering Foundation (KOSEF) through the Cell Death Disease Research Center at The Catholic University of Korea (R13-2002-005-01004-0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, C., Cho, Y., Park, C. et al. Inactivating mutations of the Siah-1 gene in gastric cancer. Oncogene 23, 8591–8596 (2004). https://doi.org/10.1038/sj.onc.1208113
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208113
Keywords
This article is cited by
-
Downregulation of Siah1 promotes colorectal cancer cell proliferation and migration by regulating AKT and YAP ubiquitylation and proteasome degradation
Cancer Cell International (2020)
-
HIPK2 suppresses tumor growth and progression of hepatocellular carcinoma through promoting the degradation of HIF-1α
Oncogene (2020)
-
Membrane-bound β-catenin degradation is enhanced by ETS2-mediated Siah1 induction in Helicobacter pylori-infected gastric cancer cells
Oncogenesis (2017)
-
SIAH proteins: critical roles in leukemogenesis
Leukemia (2013)
-
Regulators and Effectors of Siah Ubiquitin Ligases
Cell Biochemistry and Biophysics (2013)