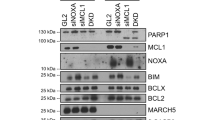
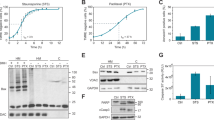
Abstract
The serine/threonine kinase mTOR, the major sensor of cell growth along the PI3K/Akt pathway, can be activated by agents acting on microtubules. Damaged microtubules induce phosphorylation of the Bcl-2 protein and lower the threshold of programmed cell death, both of which are inhibited by rapamycin. In HEK293 cells expressing Akt mutants, the level of Bcl-2 phosphorylation and the threshold of apoptosis induced by taxol or by nocodazole are significantly modified. In cells expressing dominant-negative Akt (DN-Akt), Bcl-2 phosphorylation and p70S6KThr421/Ser424 phosphorylation induced by taxol or nocodazole were significantly enhanced as compared to cells expressing constitutively active Akt (CA-Akt) and inhibited by rapamycin. Moreover, DN-Akt cells were more sensitive to antitubule agents than CA-Akt cells. In nocodazole-treated HEK293 cells sorted according to cell cycle, the p70S6KThr421/Ser424 phosphorylation was associated to the G2/M fraction. More relevant, nocodazole inhibited, in a dose–response manner, mTOR phosphorylation at Ser2448. This activity, potentiated in DN-Akt cells, was not detectable in CA-Akt cells. Our results suggest that death signals originating from damaged microtubules in G2/M can compete with G1 survival pathways at the level of mTOR. These findings have implications for cancer therapy and drug resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Abraham RT . (1998). Curr. Opin. Immunol., 10, 330–336.
Agami R and Bernards R . (2002). Cancer Lett., 177, 111–118.
Avruch J . (1998). Mol. Cell. Biochem., 182, 31–48.
Bevilacqua A, Ceriani MC, Canti GF, Asnaghi L, Gherzi R, Brewer G, Papucci L, Schiavone N, Capaccioli S and Nicolin A . (2003a). J. Biol. Chem., 278, 23451–23459.
Bevilacqua A, Ceriani MC, Capaccioli S and Nicolin A . (2003b). J. Cell Physiol., 195, 356–372.
Blagosklonny MV and Fojo T . (1999). Int. J. Cancer, 83, 151–156.
Borner C . (2003). Mol. Immunol., 39, 615–647.
Brazil DP, Park J and Hemmings BA . (2002). Cell, 111, 293–303.
Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS and Schreiber SL . (1994). Nature, 369, 756–758.
Brown EJ, Beal PA, Keith CT, Chen J, Shin TB and Schreiber SL . (1995). Nature, 377, 441–446.
Brunn GJ, Fadden P, Haystead TA and Lawrence JC . (1997). J. Biol. Chem., 272, 32547–32550.
Calastretti A, Bevilacqua A, Ceriani MC, Viganò S, Zancai P, Capaccioli S and Nicolin A . (2001a). Oncogene, 20, 6172–6180.
Calastretti A, Rancati F, Ceriani MC, Asnaghi L, Canti G and Nicolin A . (2001b). Eur. J. Cancer, 37, 2121–2128.
Chau BN and Wang JY . (2003). Nat. Rev. Cancer, 3, 130–138.
Chen J and Fang Y . (2002). Biochem. Pharmacol., 64, 1071–1077.
Church AC . (2003). QJM, 96, 91–102.
Cory S and Adams JM . (2002). Nat. Rev. Cancer, 2, 647–656.
Decary S, Decesse JT, Ogryzko V, Reed JC, Naguibneva I, Harel-Bellan A and Cremisi CE . (2002). Mol. Cell. Biol., 22, 7877–7888.
Du L, Lyle CS, Hall T and Chambers TC . (2003). Proceedings of the 94th AACR Annual Meeting.Toronto, Ontario, Canada.
Dudkin L, Dilling MB, Cheshire PJ, Harwood FC, Hollingshead M, Arbuck SG, Travis R, Sausville EA and Houghton PJ . (2001). Clin. Cancer Res., 7, 1758–1764.
Ferrara N . (2002). Nat. Rev. Cancer, 10, 795–803.
Fisher DE . (2001). Apoptosis, 6, 7–15.
Green DR and Evan GI . (2002). Cancer Cell., 1, 19–30.
Hahn WC and Weinberg RA . (2002). Nat. Rev. Cancer, 2, 331–341.
Haldar S, Basu A and Croce CM . (1997). Cancer Res., 57, 229–233.
Haldar S, Basu A and Croce CM . (1998). Cancer Res., 58, 1609–1615.
Han JW, Pearson RB, Dennis PB and Thomas G . (1995). J. Biol. Chem., 270, 396–403.
Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J and Yonezawa K . (2002). Cell, 110, 177–189.
Harada H, Andersen JS, Mann M, Terada N and Korsmeyer SJ . (2001). Proc. Natl. Acad. Sci. USA, 98, 9666–9670.
Hill MM and Hemmings BA . (2002). Pharmacol. Ther., 93, 243–251.
Jacinto E and Hall MN . (2003). Nat. Rev. Mol. Cell. Biol., 4, 117–126.
Johnstone RW, Ruefli AA and Lowe SW . (2002). Nat. Rev. Drug. Discovery, 1, 287–299.
Jordan MA and Wilson L . (1998). Curr. Opin. Cell. Biol., 10, 123–130.
Kim JE and Chen J . (2000). Proc. Natl. Acad. Sci., 97, 14340–14345.
Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P and Sabatini DM . (2002). Cell, 110, 163–175.
Koyasu S . (2003). Nat. Immunol., 4, 313–319.
Kozma SC and Thomas G . (2002). Bioessays, 24, 65–71.
Krystal GW, Sulanke G and Litz J . (2002). Mol. Cancer Ther., 1, 913–922.
Kurzrock R, Kantarjian HM, Druker BJ and Talpaz M . (2003). Ann. Intern. Med., 138, 819–830.
Laemmli UK . (1970). Nature, 227, 680–685.
Law BK, Chytil A, Dumont N, Hamilton EG, Waltner-Law ME, Aakre ME, Covington C and Moses HL . (2002). Mol. Cell. Biol., 22, 8184–8198.
Le XF, Hittelman WN, Liu J, McWatters A, Li C, Mills GB and Bast Jr RC . (2003). Oncogene, 22, 484–497.
Ling YH, Liebes L, Ng B, Buckley M, Elliott PJ, Adams J, Jiang JD, Muggia FM and Perez-Soler R . (2002). Mol. Cancer Ther., 1, 841–849.
Ling YE, Tornos C and Perez-Soler R . (1998). J. Biol. Chem., 273, 18984–18991.
Mayer TU . (2003). Trends Cell Biol., 13, 270–277.
McMahon LP, Choi KM, Lin TA, Abraham RT and Lawrence Jr JC . (2002). Mol. Cell. Biol., 22, 7428–7438.
Mosmann T . (1983). J. Immunol. Methods, 65, 55–63.
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F and Riccardi C . (1991). J. Immunol. Methods, 139, 271–279.
Ofir R, Seidman R, Rabinski T, Krup M, Yavelsky V, Weinstein Y and Wolfson M . (2002). Cell Death Differ., 9, 636–642.
Pelengaris S, Khan M and Evan G . (2002). Nat. Rev. Cancer, 2, 764–776.
Pene F, Claessens YE, Muller O, Viguie F, Mayeux P, Dreyfus F, Lacombe C and Bouscary D . (2002). Oncogene, 21, 6587–6597.
Pullen N and Thomas G . (1997). FEBS Lett., 23, 78–82.
Ruvolo PP, Deng X and May WS . (2001). Leukemia, 15, 515–522.
Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P and Snyder SH . (1994). Cell, 78, 35–43.
Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G and Abraham RT . (1995). J. Biol. Chem., 270, 815–822.
Schmelzle T and Hall MN . (2000). Cell, 103, 253–262.
Sekulic A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM and Abraham RT . (2000). Cancer Res., 60, 3504–3513.
Srivastava RK, Mi QS, Hardwick JM and Longo DL . (1999). Proc. Natl. Acad. Sci. USA, 96, 3775–3780.
Taylor WR and Stark GR . (2001). Oncogene, 20, 1803–1815.
Vincent AM and Feldman EL . (2002). Growth Horm IGF Res., 12, 193–197.
Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP and Williams RL . (2000). Mol. Cell., 6, 909–919.
Werlen G, Hausmann B, Naeher D and Palmer E . (2003). Science, 299, 1859–1863.
Yamamoto K, Ichijo H and Korsmeyer SJ . (1999). Mol. Cell. Biol., 19, 8469–8478.
Acknowledgements
We thank Dr J Chen for plasmids and critically reading the manuscript, Dr A Gulino for plasmids, Dr P Woodford for revision of the manuscript and E Fontanella for helpful FACS assistance. This work was supported by grants from AIRC, Fondazione CARIPLO, Milan, Italy; MIUR, CNR-Project Oncology, Rome, Italy.
LA and AC were supported by a fellowship from FIRC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asnaghi, L., Calastretti, A., Bevilacqua, A. et al. Bcl-2 phosphorylation and apoptosis activated by damaged microtubules require mTOR and are regulated by Akt. Oncogene 23, 5781–5791 (2004). https://doi.org/10.1038/sj.onc.1207698
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207698
Keywords
This article is cited by
-
Pathogenic mechanisms and regulatory factors involved in alcoholic liver disease
Journal of Translational Medicine (2023)
-
Therapeutic efficacy of apelin on transplanted mesenchymal stem cells in hindlimb ischemic mice via regulation of autophagy
Scientific Reports (2016)
-
Epothilone B induces apoptosis and enhances apoptotic effects of ABT-737 on human cancer cells via PI3K/AKT/mTOR pathway
Journal of Cancer Research and Clinical Oncology (2016)
-
Do prion protein gene polymorphisms induce apoptosis in non-mammals?
Journal of Biosciences (2016)
-
Resveratrol chemosensitizes HER-2-overexpressing breast cancer cells to docetaxel chemoresistance by inhibiting docetaxel-mediated activation of HER-2–Akt axis
Cell Death Discovery (2015)