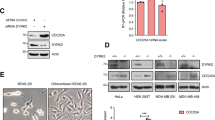
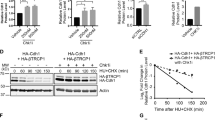
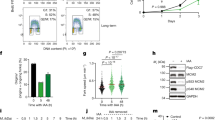
Abstract
In eukaryotic cells, control mechanisms of cell-cycle progression have evolved to accurately monitor the integrity of genetic information to be transferred to the progeny. Cdc25A phosphatase is an essential activator of cell-cycle progression and is targeted by checkpoint signals. Ubiquitylation regulates Cdc25A activity through fine tuning of its protein levels. Two different ubiquitin ligases (APC/C and SCF complex) are involved in Cdc25A turnover. While APC/C is involved in regulating Cdc25A at the exit of mitosis, SCF regulates the abundance of Cdc25A in S phase and G2. In response to DNA damage or to stalled replication, the activation of the ATM and ATR protein kinases leads to Chk1 and Chk2 activation and to Cdc25A hyperphosphorylation. These events stimulate SCF-mediated ubiquitylation of Cdc25A and its proteolysis. This contributes to delaying cell-cycle progression, thereby preventing genomic instability. Based on recent findings, we discuss the role of Cdc25A ubiquitylation and degradation in cell-cycle progression and in response to DNA damage. Moreover, we discuss the role of phosphorylation at multiple sites in triggering ubiquitylation signals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bartek J and Lukas J . (2003). Cancer Cell, 3, 421–429.
Bendjennat M, Boulaire J, Jascur T, Brickner H, Barbier V, Sarasin A, Fotedar A and Fotedar R . (2003). Cell, 114, 599–610.
Bernardi R, Liebermann DA and Hoffman B . (2000). Oncogene, 19, 2447–2454.
Blomberg I and Hoffmann I . (1999). Mol. Cell. Biol., 19, 6183–6194.
Broggini M, Buraggi G, Brenna A, Riva L, Codegoni AM, Torri V, Lissoni AA, Mangioni C and D'Incalci M . (2000). Anticancer Res., 20, 4835–4840.
Busino L, Donzelli M, Chiesa M, Guardavaccaro D, Ganoth D, Dorrello NV, Hershko A, Pagano M and Draetta GF . (2003). Nature, 426, 87–91.
Cangi MG, Cukor B, Soung P, Signoretti S, Moreira Jr G, Ranashinge M, Cady B, Pagano M and Loda M . (2000). J. Clin. Invest., 106, 753–761.
Carrano AC, Eytan E, Hershko A and Pagano M . (1999). Nat. Cell Biol., 1, 193–199.
Cenciarelli C, Chiaur DS, Guardavaccaro D, Parks W, Vidal M and Pagano M . (1999). Curr. Biol., 9, 1177–1179.
Clucas C, Cabello J, Bussing I, Schnabel R and Johnstone IL . (2002). EMBO J., 21, 665–674.
Di Leonardo A, Linke SP, Clarkin K and Wahl GM . (1994). Genes Dev., 8, 2540–2551.
Donzelli M and Draetta GF . (2003). EMBO Rep., 4, 671–677.
Donzelli M, Squatrito M, Ganoth D, Hershko A, Pagano M and Draetta GF . (2002). EMBO J., 21, 4875–4884.
Dunphy WG and Kumagai A . (1991). Cell, 67, 189–196.
Falck J, Mailand N, Syljuasen RG, Bartek J and Lukas J . (2001). Nature, 410, 842–847.
Feldman RM, Correll CC, Kaplan KB and Deshaies RJ . (1997). Cell, 91, 221–230.
Galaktionov K and Beach D . (1991). Cell, 67, 1181–1194.
Galaktionov K, Chen X and Beach D . (1996). Nature, 382, 511–517.
Galaktionov K, Lee AK, Eckstein J, Draetta G, Meckler J, Loda M and Beach D . (1995). Science, 269, 1575–1577.
Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, Rideout WM, Bronson RT, Gardner H and Sicinski P . (2003). Cell, 114, 431–443.
Glotzer M, Murray AW and Kirschner MW . (1991). Nature, 349, 132–138.
Goloudina A, Yamaguchi H, Chervyakova DB, Appella E, Fornace Jr AJ and Bulavin DV . (2003). Cell Cycle, 2, 473–478.
Guardavaccaro D, Kudo Y, Boulaire J, Barchi M, Busino L, Donzelli M, Margottin-Goguet F, Jackson PK, Yamasaki L and Pagano M . (2003). Dev. Cell, 4, 799–812.
Hassepass I, Voit R and Hoffmann I . (2003). J. Biol. Chem., 278, 29824–29829.
Hernandez S, Bessa X, Bea S, Hernandez L, Nadal A, Mallofre C, Muntane J, Castells A, Fernandez PL, Cardesa A and Campo E . (2001). Lab. Invest., 81, 465–473.
Hochstrasser M . (1996). Annu. Rev. Genet., 30, 405–439.
Hoffmann I, Draetta G and Karsenti E . (1994). EMBO J., 13, 4302–4310.
Ito Y, Yoshida H, Nakano K, Kobayashi K, Yokozawa T, Hirai K, Matsuzuka F, Matsuura N, Kakudo K, Kuma K and Miyauchi A . (2002). Br. J. Cancer, 86, 1909–1913.
Jinno S, Suto K, Nagata A, Igarashi M, Kanaoka Y, Nojima H and Okayama H . (1994). EMBO J., 13, 1549–1556.
Kaneko YS, Watanabe N, Morisaki H, Akita H, Fujimoto A, Tominaga K, Terasawa M, Tachibana A, Ikeda K, Nakanishi M and Kaneko Y . (1999). Oncogene, 18, 3673–3681.
Koepp DM, Schaefer LK, Ye X, Keyomarsi K, Chu C, Harper JW and Elledge SJ . (2001). Science, 294, 173–177.
Kramer ER, Scheuringer N, Podtelejnikov AV, Mann M and Peters JM . (2000). Mol. Cell. Biol., 11, 1555–1569.
Listovsky T, Zor A, Laronne A and Brandeis M . (2000). Exp. Cell Res., 255, 184–191.
Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X and He X . (2002). Cell, 108, 837–847.
Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, Donehower LA and Elledge SJ . (2000). Genes Dev., 14, 1448–1459.
Lukas C, Bartkova J, Latella L, Falck J, Mailand N, Schroeder T, Sehested M, Lukas J and Bartek J . (2001). Cancer Res., 61, 4990–4993.
Lukas C, Sorensen CS, Kramer E, Santoni-Rugiu E, Lindeneg C, Peters JM, Bartek J and Lukas J . (1999). Nature, 401, 815–818.
Mailand N, Falck J, Lukas C, Syljuasen RG, Welcker M, Bartek J and Lukas J . (2000). Science, 288, 1425–1429.
Mailand N, Podtelejnikov AV, Groth A, Mann M, Bartek J and Lukas J . (2002). EMBO J., 21, 5911–5920.
Margottin-Goguet F, Hsu JY, Loktev A, Hsieh HM, Reimann JD and Jackson PK . (2003). Dev. Cell, 4, 813–826.
Melchionna R, Chen XB, Blasina A and McGowan CH . (2000). Nat. Cell Biol., 2, 762–765.
Molinari M, Mercurio C, Dominguez J, Goubin F and Draetta GF . (2000). EMBO Rep., 1, 71–79.
Nash P, Tang X, Orlicky S, Chen Q, Gertler FB, Mendenhall MD, Sicheri F, Pawson T and Tyers M . (2001). Nature, 414, 514–521.
Orlicky S, Tang X, Willems A, Tyers M and Sicheri F . (2003). Cell, 112, 243–256.
Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, Barbero JL, Malumbres M and Barbacid M . (2003). Nat. Genet., 35, 25–31.
Parisi T, Beck AR, Rougier N, McNeil T, Lucian L, Werb Z and Amati B . (2003). EMBO J., 22, 4794–4803.
Peters JM . (2002). Mol. Cell, 9, 931–943.
Peters JM . (2003). Mol. Cell, 11, 1420–1421.
Pfleger CM and Kirschner MW . (2000). Genes Dev., 14, 655–665.
Pines J . (1999). Nat. Cell Biol., 1, E73–E79.
Polakis P . (1999). Curr. Opin. Genet. Dev., 9, 15–21.
Reimann JD, Freed E, Hsu JY, Kramer ER, Peters JM and Jackson PK . (2001). Cell, 105, 645–655.
Russell P and Nurse P . (1986). Cell, 45, 145–153.
Sexl V, Diehl JA, Sherr CJ, Ashmun R, Beach D and Roussel MF . (1999). Oncogene, 18, 573–582.
Shimuta K, Nakajo N, Uto K, Hayano Y, Okazaki K and Sagata N . (2002). EMBO J., 21, 3694–3703.
Skowyra D, Craig KL, Tyers M, Elledge SJ and Harper JW . (1997). Cell, 91, 209–219.
Sorensen CS, Syljuasen RG, Falck J, Schroeder T, Ronnstrand L, Khanna KK, Zhou BB, Bartek J and Lukas J . (2003). Cancer Cell, 3, 247–258.
Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O and Reed SI . (2001). Nature, 413, 316–322.
Takai H, Tominaga K, Motoyama N, Minamishima YA, Nagahama H, Tsukiyama T, Ikeda K, Nakayama K and Nakanishi M . (2000). Genes Dev., 14, 1439–1447.
Tsvetkov LM, Yeh KH, Lee SJ, Sun H and Zhang H . (1999). Curr. Biol., 9, 661–664.
Verma R, Feldman RM and Deshaies RJ . (1997). Mol. Cell. Biol., 8, 1427–1437.
Vigo E, Muller H, Prosperini E, Hateboer G, Cartwright P, Moroni MC and Helin K . (1999). Mol. Cell. Biol., 19, 6379–6395.
Winston JT, Koepp DM, Zhu C, Elledge SJ and Harper JW . (1999). Curr. Biol., 9, 1180–1182.
Wu G, Xu G, Schulman BA, Jeffrey PD, Harper JW and Pavletich NP . (2003). Mol. Cell, 11, 1445–1456.
Xiao Z, Chen Z, Gunasekera AH, Sowin TJ, Rosenberg SH, Fesik S and Zhang H . (2003). J. Biol. Chem., 278, 21767–21773.
Xu X, Yamamoto H, Sakon M, Yasui M, Ngan CY, Fukunaga H, Morita T, Ogawa M, Nagano H, Nakamori S, Sekimoto M, Matsuura N and Monden M . (2003). Clin. Cancer Res., 9, 1764–1772.
Zachariae W, Schwab M, Nasmyth K and Seufert W . (1998). Science, 282, 1721–1724.
Zhao H, Watkins JL and Piwnica-Worms H . (2002). Proc. Natl. Acad. Sci. USA, 99, 14795–14800.
Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, Chu C, Koepp DM, Elledge SJ, Pagano M, Conaway RC, Conaway JW, Harper JW and Pavletich NP . (2002). Nature, 416, 703–709.
Zhou BB and Elledge SJ . (2000). Nature, 408, 433–439.
Zou L and Stillman B . (1998). Science, 280, 593–596.
Acknowledgements
We thank CL Attwooll and AP Bracken for critically reading the manuscript. Work in the authors' laboratory is supported by grants from AIRC, FIRC and Telethon.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Busino, L., Chiesa, M., Draetta, G. et al. Cdc25A phosphatase: combinatorial phosphorylation, ubiquitylation and proteolysis. Oncogene 23, 2050–2056 (2004). https://doi.org/10.1038/sj.onc.1207394
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207394
Keywords
This article is cited by
-
SRSF10 stabilizes CDC25A by triggering exon 6 skipping to promote hepatocarcinogenesis
Journal of Experimental & Clinical Cancer Research (2022)
-
A novel CDC25A/DYRK2 regulatory switch modulates cell cycle and survival
Cell Death & Differentiation (2022)
-
Ubiquitin signaling in cell cycle control and tumorigenesis
Cell Death & Differentiation (2021)
-
The REGγ inhibitor NIP30 increases sensitivity to chemotherapy in p53-deficient tumor cells
Nature Communications (2020)
-
Ataxia telangiectasia and Rad3-related inhibitors and cancer therapy: where we stand
Journal of Hematology & Oncology (2019)