Abstract
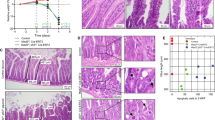
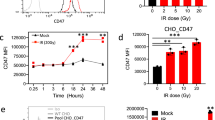
MBD4 was originally identified through its methyl binding domain, but has more recently been characterized as a thymine DNA glycosylase that interacts with the mismatch repair (MMR) protein MLH1. In vivo, MBD4 functions to reduce the mutability of methyl-CpG sites in the genome and mice deticient in MBD4 show increased intestinal tumorigenesis on an ApcMin/+ background. As MLH1 and other MMR proteins have been functionally linked to apoptosis, we asked whether MBD4 also plays a role in mediating the apoptotic response within the murine small intestine. Mice deficient for MBD4 showed significantly reduced apoptotic responses 6 h following treatment with a range of cytotoxic agents including γ-irradiation, cisplatin, temozolomide and 5-fluorouracil (5-FU). This leads to increased clonogenic survival in vivo in Mbd4−/− mice following exposure to either 5-FU or cisplatin. We next analysed the apoptotic response to 5-FU and temozolomide in doubly mutant Mbd4−/−, Mlh1−/− mice but observed no additive decrease. The results imply that MBD4 and MLH1 lie in the same pathway and therefore that MMR-dependent apoptosis is mediated through MBD4. MBD4 deficiency also reduced the normal apoptotic response to γ-irradiation, which we show is independent of Mlh1 status (at least in the murine small intestine), so suggesting that the reliance upon MBD4 may extend beyond MMR-mediated apoptosis. Our results establish a novel functional role for MBD4 in the cellular response to DNA damage and may have implications for its role in suppressing neoplasia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bader S, Walker M and Harrison D . (2000). Br. J. Cancer, 83, 1646–1649.
Bader S, Walker M, Heindrich B, Bird A, Bird C, Hooper M and Wyllie A . (1999). Oncogene, 18, 8044–8047.
Bellacosa A . (2001). J. Cell. Physiol., 187, 137–144.
Bellacosa A, Cicchillitti L, Schepis F, Riccio A, Yeung AT, Matsumoto Y, Golemis EA, Genuardi M and Neri G . (1999). Proc. Natl. Acad. Sci. USA, 96, 3969–3975.
Buermeyer AB, Deschennes SM, Baker SM and Liskay RM . (1999). Annu. Rev. Genet., 33, 533–564.
Drummond JT and Bellacosa A . (2001). Nucleic Acid Res., 29, 2234–2243.
Fishel R . (1999). Nat. Med., 5, 1239–1241.
Hendrich B and Bird A . (1998). Mol. Cell. Biol., 18, 6538–6547.
Hendrich B, Hardeland U, Ng HH, Jiricny J and Bird A . (1999). Nature, 401, 301–304.
Hendry JH, Cai WB, Roberts SA and Potten CS . (1997). Radiat. Res., 148, 254–259.
Herr I, Wilhelm D, Bohler T, Angel P and Debatin K-M . (1997). EMBO J., 16, 6200–6208.
Ijiri K and Potten CS . (1983). Br. J. Cancer, 47, 175–185.
Karran P and Bignami M . (1992). Nucleic Acid Res., 20, 2933–2940.
Meyers M, Wagner MW, Hwang HS, Kinsella TJ and Boothman DA . (2001). Cancer Res., 61, 5193–5201.
Micheau O, Solary E, Hammann A and Dimanche-Boitrel MT . (1999). J. Biol. Chem., 274, 7987–7992.
Millar CB, Guy J, Sansom OJ, Selfridge J, MacDougall E, Hendrich B, Keightley PD, Bishop SM, Clarke AR and Bird A . (2002). Science, 297, 403–405.
Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C and Kolesnick R . (2001). Science, 293, 293–297.
Petronzelli F, Riccio A, Markham GD, Seeholzer SH, Genuardi M, Karbowski M, Yeung AT, Matsumoto Y and Bellacosa A . (2000). J. Biol. Chem., 185, 473–480.
Potten CS . (1990). Int. J. Radiat. Biol., 58, 925–973.
Pritchard DM, Potten CS and Hickman JA . (1998). Cancer Res., 58, 5453–5465.
Prolla TA, Baker SM, Harris AC, Tsao EL, Yao X, Bronner CE, Zheng BH, Gordon M, Reneker J, Amheim N, Shibata D, Bradley A and Liskay RM . (1998). Nat. Genet., 18, 276–279.
Riccio A, Aaltonen LA, Godwin AK, Loukola A, Percesepe A, Salovaara R, Masciullo V, Genuardi M, Paravatou-Petsotas M, Bassi DE, Ruggeri BA, Klein-Szanto AJP, Testa JR, Neri G and Bellacosa A . (1999). Nat. Genet., 23, 266–268.
Sansom OJ and Clarke AR . (2000). Mutation Res.-Fundam. Mol. Mech. Mutagen., 452, 149–162.
Sansom OJ and Clarke AR . (2002). Oncogene, 21, 5934–5399.
Sansom OJ, Toft NJ, Winton DJ and Clarke AR . (2001). Oncogene, 20, 3580–3584.
Screaton RA, Kiessling S, Sansom OJ, Millar CB, Maddison K, Bird A, Clarke AR, Frisch SM . (2003). Proc. Natl. Acad. USA, 100, 5211–5216.
Toft NJ, Winton DJ, Kelly J, Howard LA, Dekker M, Riele HT, Arends MJ, Wyllie AH, Margison GP and Clarke AR . (1999). Proc. Natl. Acad. USA, 96, 3911–3915.
Acknowledgements
We thank Nathan Hill for maintenance of animal stocks and Steven Frisch for helpful discussions. This work was supported by Grants from the Cancer Research UK and the Wellcome trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sansom, O., Zabkiewicz, J., Bishop, S. et al. MBD4 deficiency reduces the apoptotic response to DNA-damaging agents in the murine small intestine. Oncogene 22, 7130–7136 (2003). https://doi.org/10.1038/sj.onc.1206850
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1206850
Keywords
This article is cited by
-
Differential molecular response in mice and human thymocytes exposed to a combined-dose radiation regime
Scientific Reports (2022)
-
Endogenous c-Myc is essential for p53-induced apoptosis in response to DNA damage in vivo
Cell Death & Differentiation (2014)
-
MBD4 gene is associated with rheumatoid arthritis in Chinese patients in Taiwan
Rheumatology International (2012)
-
Epithelial Pten is dispensable for intestinal homeostasis but suppresses adenoma development and progression after Apc mutation
Nature Genetics (2008)
-
Mechanisms of Disease: methyl-binding domain proteins as potential therapeutic targets in cancer
Nature Clinical Practice Oncology (2007)