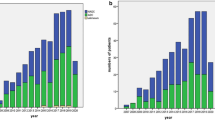
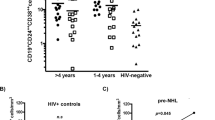
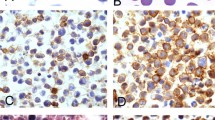
Abstract
Since the emergence of the HIV pandemic, a close association between HIV infection and the development of a selected group of cancers has been acknowledged. The introduction of highly active antiretroviral therapy, however, has had a dramatic impact on the incidences of several AIDS-defining malignancies. This suggests the possibility of a direct and indirect role of HIV in HIV-related tumor genesis. The aim of this paper is to review the pathology of AIDS-related malignancies, taking into account the pathogenetic mechanisms and their potential for improving the treatment of these tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Albini A, Barillari G, Benelli R, Gallo RC and Ensoli B . (1995). Proc. Natl. Acad. Sci. USA, 92, 4838–4842.
Altavilla G, Trabanelli C, Merlin M, Caputo A, Lanfred M, Barbanti Brodand G and Corallini A . (1999). Am. J. Pathol., 154, 1231–1244.
Ambinder RF . (2001). Eur. J. Cancer, 37, 1209–1216.
Ambrosino C, Palmieri C, Puca A, Trimboli F, Schiavone M, Olimpico F, Ruocco MR, Di Leva F, Toriello M, Quinto I, Venuta S and Scala G . (2002). J. Biol. Chem., 277, 31448–31458.
Babcock GJ and Thorley-Lawson DA . (2000). Proc. Natl. Acad. Sci. USA, 97, 12250–12255.
Barillari G, Buonaguro L, Fiorelli V, Hoffman J, Michaels F, Gallo RC and Ensoli B . (1992). J. Virol., 66, 7159–7167.
Barillari G and Ensoli B . (2002). Clin. Microbiol. Rev., 15, 310–326.
Bedi GC, Westra WH, Farzadegan H, Pitha PM and Sidransky D . (1995). Nat. Med., 1, 65–68.
Beral V, Peterman TA, Berkelman RL and Jaffe HW . (1990). Lancet, 335, 123–128.
Boccalon M, Tirelli U, Sopracordevole F and Vaccher E . (1996). Eur. J. Cancer, 32A, 2212–2217.
Boshoff C and Weiss R . (2002). Nat. Rev. Cancer, 5, 373–382.
Cannon M and Cesarman E . (2000). Semin. Oncol., 27, 409–419.
Carbone A . (2003). Lancet Oncol., 4, 22–29.
Cesarman E, Chang Y, Moore PS, Said JW and Knowles DM . (1995). N. Engl. J. Med., 332, 1186–1191.
Cesaman E and Knowles DM . (1997). Semin. Diagn. Pathol., 14, 54–66.
Chang HK, Gallo RC and Ensoli B . (1995). J. Biomed. Sci., 2, 189–202.
Cinti C, Leoncini L, Nyongo A, Ferrari F, Lazzi S, Bellan C, Vatti R, Zamparelli A, Cevenini G, Tosi GM, Claudio PP, Maraldi NM, Tosi P and Giordano A . (2000). Am. J. Pathol., 156, 751–760.
Clarke B and Chetty R . (2002). Mol. Pathol., 55, 19–24.
Claudio PP, Caputi M and Giordano A . (2000a). Clin. Cancer Res., 6, 754–764.
Claudio PP, Howard CM, Pacilio C, Cinti C, Romano G, Minimo C, Maraldi NM, Minna JD, Gelbert L, Leoncini L, Tosi GM, Micheli P, Caputi M, Giordano GG and Giordano A . (2000b). Cancer Res., 60, 372–382.
Corallini A, Altavilla G, Pozzi L, Bignozzi F, Negrini M, Rimessi P, Gualandi F and Barbanti-Brodano G . (1993). Cancer Res., 53, 5569–5575.
Dal Maso L, Serraino D and Franceschi S . (2001). Eur. J. Cancer., 37, 1188–1201.
De Falco G, Bellan C, Lazzi S, Claudio PP, La Sala D, Cinti C, Tosi P, Giordano A and Leoncini L . (2003). Oncogene, in press.
Dalgleish AG and O'Byrne KJ . (2002). Adv. Cancer Res., 84, 231–276.
De La Fuente C, Santiago F, Deng L, Eadie C, Ziberman I, Kehn K, Maddukuri A, Baylor S, Wu K, Lee CG, Pumfery A and Kashanchi F . (2002). BMC Biochem., 3, 14.
De Luca A, Machachlan TK, Bagella L, Dean C, Howard CM, Claudio PP, Baldi A, Khalili K and Giordano A . (1997). J. Biol. Chem., 272, 20971–20974.
D'Oliveira JJ and Torres FO . (1972). Cancer, 30, 553–561.
Du MQ, Liu H, Diss TC, Ye H, Hamoudi RA, Dupin, N, Meignin V, Oksenhendler E, Boshoff C and Isaacson PG . (2001). Blood, 97, 2130–2136.
Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, van Marck E, Salmon D, Gorin I, Escande, JP, Weiss RA, Alitalo K and Boshoff C . (1999). Proc. Natl. Acad. Sci. USA, 96, 4546–4551.
Dyson N, Howley PM, Munger K and Harlow E . (1989). Science, 243, 934–937.
Ellis M, Chew YP, Fallis L, Freddersdorf S, Boshoff C, Weiss RA, Lu X and Mittnacht S . (1999). EMBO J., 18, 644–653.
Ensoli B, Barillari G, Salahuddin SZ, Gallo RC and Wong-Staal F . (1990). Nature, 345, 84–86.
Ensoli B, Gendelman R, Markham P, Fiorelli V, Colombini S, Raffeld M, Cafaro A, Chang HK, Brady JN and Gallo RC . (1994). Nature, 371, 674–680.
Ensoli B, Nakamura S, Salahuddin SZ, Biberfeld P, Larsson L, Beaver B, Wong-Staal F and Gallo RC . (1989). Science, 243, 223–226.
Fattaey AR, Harlow E and Helin K . (1993). Mol. Cell Biol., 13, 7267–7772.
Fiorelli V, Gendelman R, Sirianni MC, Chang HK, Colombini S, Markham PD, Monini P, Sonnabend J, Pintus A, Gallo RC and Ensoli B . (1998). Blood, 91, 956–967.
Gage JR, Meyers C and Wettstein FO . (1990). J. Virol., 64, 723–730.
Gaidano G, Carbone A and Dalla Favera R . (1998). Am. J. Pathol., 152, 623–630.
Gaidano G, Pasqualucci L, Capello D, Berra E, Deambrogi C, Rossi D, Larocca LM, Gloghini A, Carbone A and Dalla-Favera R . (2003). Blood, Apr. 24 (EPUB ahead of print).
Gates AE and Kaplan LD . (2002). Oncology (Huntingt), 16, 657–665, discussion 665, 668–670.
Godden-Kent D, Talbot SJ, Boshoff C, Chang Y, Moore P, Weiss RA and Mittnacht S . (1997). J. Virol., 71, 4193–4198.
Hengge UR, Ruzicka T, Tyring SK, Stuschke M, Roggendorf M, Schwartz RA and Seeber S . (2002). Lancet Infect. Dis., 2, 344–352.
Jussila L, Valtola R, Partanen TA, Salven P, Heikkila P, Matikainen MT, Renkonen R, Kaipainen A, Detmar M, Tschachler E, Alitalo R and Alitalo K . (1998). Cancer Res., 58, 1599–1604.
Kaposi M . (1872). Arch. Dermatologie Syphillis, 265–273.
Kashanchi F, Agbottah ET, Pise-Masison CA, Mahieu R, Duvall J, Kumar A and Brady NJ . (2000). J. Virol, 74, 652–660.
Kersemaekers AM, Hermans J, Fleuren GJ and van de Vijver MJ . (1998). Br. J. Cancer, 77, 192–200.
Klein G . (1994). Cell, 77, 791–793.
Kim CM, Vogel J, Jay G and Rhim JS . (1992). Oncogene, 7, 1525–1529.
Knowles DM . (1999). Mod. Pathol., 12, 200–217.
Kundu M, Guermah M, Roeder RG, Amini S and Khalili K . (1997). J. Biol. Chem., 272, 29468–29474.
Kundu RK, Sangiorgi F, Wu LY, Pattengale PK, Hinton DR, Gill PS and Maxson R . (1999). Blood, 94, 275–282.
Kundu M, Sharma S, De Luca A, Giordano A, Rappaport J, Khalili K and Amini S . (1998). J. Biol. Chem., 273, 8130–8136.
Laman H, Coverley D, Krude T, Laskey R and Jones N . (2001). Mol. Cell. Biol., 21, 624–635.
Lazzi S, Bellan C, De Falco G, Cinti C, Ferrari F, Nyongo A, Claudio PP, Tosi GM, Vatti R, Gloghini A, Carbone A, Giordano A, Leoncini L and Tosi P . (2002). Hum. Pathol., 33, 723–731.
Li CJ, Wang C, Friedman DJ and Pardee AB . (1995). Proc. Natl. Acad. Sci. USA, 92, 5461–5464.
Miles SA, Rezai AR, Salazar-Gonzalez JF, Vander Meyden M, Stevens RH, Logan DM, Mitsuyasu RT, Taga T, Hirano T and Kishimoto T et al. (1990). Proc. Natl. Acad. Sci. USA, 87, 4068–4072.
Mullokandov MR, Kholodilov NG, Atkin NB, Burk RD, Johnson AB and Klinger HP . (1996). Cancer Res., 56, 197–205.
Noonan D and Albini A . (2000). Adv. Pharmacol., 48, 229–250.
Olweny CL . (1984). IARC Sci. Publ., 63, 543–548.
Prakash O, Tang ZY, He YE, Ali MS, Coleman R, Gill J, Farr G and Samaniego F . (2000). J. Natl. Cancer Inst., 92, 721–728.
Preudhomme C, Dervite I, Wattel E, Vanrumbeke M, Flactif M, Lai JL, Hecquet B, Coppin MC, Nelken B and Gosselin B . (1995). J. Clin. Oncol., 13, 812–820.
Rabkin CS . (2001). Eur. J. Cancer., 37, 1316–1319.
Roth WK, Brandstetter H and Stuizl M . (1992). AIDS, 6, 895–913.
Rubartelli A, Poggi A, Sitia R and Zocchi MR . (1998). Immunol. Today, 19, 543–545.
Salahuddin SZ, Nakamura S, Biberfeld P, Kaplan MH, Markham PD, Larsson L and Gallo RC . (1988). Science, 242, 430–433.
Schulz TF . (2001). Eur. J. Cancer, 37, 1217–1226.
Schulz TF, Sheldon J and Greensill J . (2002). Virus Res., 82, 115–126.
Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay MF, Clauvel JP, Raphael M and Degos L et al. (1995). Blood, 86, 1276–1280.
Stiegler P, Kasten M and Giordano A . (1998). J. Cell Biochem. Suppl., 30–31, 30–36.
Thorley-Lawson DA . (2001). Nat. Rev. Immunol., 1, 75–82.
Tirelli U, Bernardi D, Spina M and Vaccher E . (2002). Crit. Rev. Oncol. Hematol., 41, 299–315.
Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, Kubo H, Thurston G, McDonald DM, Achen MG, Stacker SA and Alitalo K . (2001). EMBO J., 20, 1223–1231.
Vogel J, Hinrichs SH, Reynolds RK, Luciw PA and Jay G . (1988). Nature, 335, 606–611.
Wallace SV and Carlin EM . (2001). Int. J. STD AIDS, 12, 283–285.
Wistuba II, Syed S, Behrens C, Duong M, Milchgrub S, Muller CY, Jagirdar J and Gazdar AF . (1999). Gynecol. Oncol., 74, 519–526.
Zauli G, Gibellini D, Caputo A, Bassini A, Negrini M, Monne M, Mazzoni M and Capitani S . (1995). Blood, 86, 3823–3834.
Acknowledgements
We acknowledge the help of Emma Thorley in editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is cited by
-
Long-term remission of AIDS-related primary central nervous system lymphoma in a patient under antiretroviral therapy: a case report and review of the literature
AIDS Research and Therapy (2021)
-
A review of the pattern of AIDS defining, HIV associated neoplasms and premalignant lesions diagnosed from 2000–2011 at Kenyatta National Hospital, Kenya
Infectious Agents and Cancer (2015)
-
E2F4 plays a key role in Burkitt lymphoma tumorigenesis
Leukemia (2012)
-
Urologic complications of HIV and AIDS
Nature Clinical Practice Urology (2009)
-
Growth regulation of simian and human AIDS-related non-Hodgkin's lymphoma cell lines by TGF-β1 and IL-6
BMC Cancer (2007)