Abstract
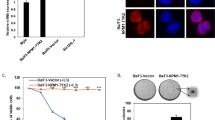
A highly specific t(8;13)(p11;q12) translocation has been consistently identified in bone marrow cells from patients with an atypical myeloproliferative disease that is associated with peripheral blood eosinophila and T- or B-cell leukemias. In all patients analysed to date, the translocation event results in a chimeric gene in which the atypical zinc-finger domain of ZNF198 is fused to the N-terminal end of the catalytic domain of the FGFR1 receptor tyrosine kinase. To understand more about the consequences of this rearrangement we have investigated the normal function of the ZNF198 gene. Using yeast two-hybrid analysis we identified HHR6 as a protein binding partner and confirmed this using immunoprecipitation studies. The ZNF198/FGFR1 fusion protein also binds to HHR6. We demonstrate here that the human RAD18 is also present in the ZNF198/HHR6 protein complex, although it does not coimmunoprecipitate with the fusion kinase. Cells expressing the fusion kinase gene show a marked increased sensitivity to UVB irradiation, suggesting that it acts in a dominant-negative way to affect DNA repair. These observations support the idea that ZNF198, through its interaction with HHR6 and RAD18, may be involved in the DNA repair process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Abruzzo LV, Jaffe ES, Cotelingam JD, Whang-Peng J, Del Duca V and Medeiros LJ . (1992). Am. J. Surg. Pathol., 16, 236–245.
Bailly V, Lamb J, Sung P and Prakash S . (1994). Genes Dev., 8, 811–820.
Bailly V, Lauder S, Prakash S and Prakash L . (1997a). J. Biol. Chem., 272, 23360–23365.
Bailly V, Prakash S and Prakash L . (1997b). Mol. Cell. Biol., 17, 4536–4543.
Baumann H, Kunapuli P, Tracy E and Cowell JK . 2003. J. Biol. Chem., in press.
Chernova O, Still I, Kalaycio M, Hoeltge G and Cowell JK . (1998). Genes Chromosomes Cancer, 21, 160–165.
Fields S and Song OK . (1989). Nature, 340, 245–246.
Harper JW, Adami GR, Wel N, Keyomarsi K and Elledge SJ . (1993). Cell, 75, 805–816.
Hoege C, Pfander B, Moldovan G-L, Pyrowalakis G and Jentsch S . (2002). Nature, 419, 135–141.
Jentsch S, McGrath JP and Varshavsky A . (1987). Nature, 329, 131–134.
Kempski H, MacDonald D, Michalski AJ, Roberts T, Goldman JM, Cross CP and Cowell JK . (1995). Genes Chromosomes Cancer, 12, 283–287.
Koken MH, Reynolds P, Jaspers-Dekker I, Prakash L, Prakash S, Bootsma D and Hoeijmakers JH . (1991). Proc. Natl. Acad. Sci. USA, 88, 8865–8869.
Lawrence C . (1994). Bioessays, 16, 253–258.
Mackay JP and Crossley M . (1998). Trends Biochem. Sci., 23, 1–4.
Ollendorff V, Guasch G, Isnardon D, Galindo R, Birnbaum D and Pebusque MJ . (1999). J. Biol. Chem., 274, 26922–26930.
Popovici C, Zhang B, Grégoire MJ, Jonveaux P, Lafage-Pochitaloff M, Birnbaum D and Pébusque MJ . (1999). Blood, 93, 1381–1389.
Prakash L . (1981). Mol. Gen. Genet., 184, 471–478.
Shekhar MP, Lyakhovich A, Visscher DW, Heng H and Kondrat N . (2002). Cancer Res., 62, 2115–2124.
Smedley D, Demiroglu A, Abdul-Rauf M, Heath C, Cooper C, Shipley J and Cross NCP . (1999). Neoplasia, 1, 249–355.
Smedley D, Hamoudi R, Clark J, Warren W, Abdul-Rauf M, Somers G, Venter D, Fagan K, Cooper C and Shipley J . (1998). Hum. Mol. Genet., 7, 637–642.
Still IH, Chernova O, Hurd D, Stone RM and Cowell JK . (1997). Blood, 90, 3136–3141.
Still IH and Cowell JK . (1998). Blood, 92, 1456–1458.
Sung P, Prakash S and Prakash L . (1988). Genes Dev., 2, 1476–1485.
Sun Z and Allis DC . (2002). Nature, 418, 104–108.
Tam JP . (1988). Proc. Natl. Acad. Sci. USA, 85, 5409–5413.
Tateishi S, Sakuraba Y, Masuyama S, Inoue H and Yamaizumi M . (2000). Proc. Natl. Acad. Sci. USA, 97, 7927–7932.
Van der Laan R, Roest HP, Hoogerbrugge JW, Smit EM, Slater R, Baarends WM, Hoeijmakers JH and Grootegoed JA . (2000). Genomics, 69, 86–94.
Xiao S, Nalabolu SR, Aster JC, Ma J, Abruzzo L, Jaffe ES, Stone R, Weissman SM, Hudson TJ and Fletcher JA . (1998). Nat. Genet., 18, 84–87.
Xin H, Lin W, Sumanasekera W, Zhang Y, Wu X and Wang Z . (2000). Nucleic Acids Res., 28, 2847–2854.
Acknowledgements
This work was supported by Grant CA76167 from the National Institutes of Health and in part by the Roswell Park Cancer Center Support Grant (P30 CA 16056-26).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kunapuli, P., Somerville, R., Still, I. et al. ZNF198 protein, involved in rearrangement in myeloproliferative disease, forms complexes with the DNA repair-associated HHR6A/6B and RAD18 proteins. Oncogene 22, 3417–3423 (2003). https://doi.org/10.1038/sj.onc.1206408
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1206408