Abstract
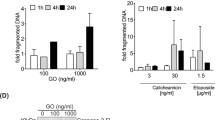
Smac (or DIABLO) is a recently identified, novel proapoptotic molecule, which is released from mitochondria into the cytosol during apoptosis. Smac functions by eliminating the caspase-inhibitory properties of the inhibitors of apoptosis proteins (IAP), particularly XIAP. In this study, we stably transfected both full-length (FL) and mature (MT) Smac genes into the K562 and CEM leukaemic cell lines. Both FL and MT Smac transfectants increased the sensitivity of leukaemic cells to UV light-induced apoptosis and the activation of caspase-9 and caspase-3. Purified cytosol from the mature Smac transfectants, or the addition of human recombinant Smac protein or N-7 peptide into nontransfected cytosol, showed an increased sensitivity to cytochrome c-induced activation of caspase-3. The mature Smac enhanced the susceptibility of both K562 and CEM cells to TRAIL-induced apoptosis. Overexpression of the mature Smac protein also inhibited proliferation, as detected by reduced colony formation and Ki-67 expression in leukaemic cells. Cell cycle analysis revealed that Smac transfectants displayed significant G0/G1 arrest and reduction in 5-bromo-2′-deoxyuridine (BrdU) incorporation. Smac sensitized human acute myeloid leukaemia blasts to cytochrome c-induced activation of caspase-3. However, Smac failed to overcome Apaf-1-deficiency-mediated resistance to cytochrome c in primary leukaemic blasts. In summary, this study reveals that Smac/DIABLO exhibits a potential role in increasing apoptosis and suppressing proliferation in human leukaemic cells. Importantly, it also indicates that it is crucial to evaluate the levels of Apaf-1 and XIAP proteins in patient samples before using Smac peptide therapy in the treatment of human leukaemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Altieri DC . (2001). Trends Mol. Med., 7, 542–547.
Carson JP, Behnam M, Sutton JN, Du C, Wang X, Hunt DF, Weber MJ and Kulik G . (2002). Cancer Res., 62, 18–23.
Chai J, Du C, Wu JW, Kyin S, Wang X and Shi Y . (2000). Nature, 406, 855–862.
Deng Y, Lin Y and Wu X . (2002). Genes Dev., 16, 33–45.
Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS and Reed JC . (1998). EMBO J., 17, 2215–2223.
Du C, Fang M, Li Y, Li L and Wang X . (2000). Cell, 102, 33–42.
Ekert PG, Silke J, Hawkins CJ, Verhagen AM and Vaux DL . (2001). J. Cell Biol., 152, 483–490.
Fulda S, Kufer MU, Meyer E, van Valen F, Dockhorn-Dworniczak B and Debatin KM . (2001). Oncogene, 20, 5865–5877.
Fulda S, Wick W, Weller M and Debatin KM . (2002). Nat. Med., 8, 808–815.
Gerlach C, Kubbutat M, Schwab V, Key G, Flad HD and Gerdes J . (1998). Lab. Invest., 78, 129–130.
Green DR and Reed JC . (1998). Science, 281, 1309–1312.
Guo F, Nimmanapalli R, Paranawithana S, Wittman S, Griffin D, Bali P, O'Bryan E, Fumero C, Wang HG and Bhalla K . (2002). Blood, 99, 3419–3426.
Hegde R, Srinivasula SM, Zhang Z, Wassell R, Mukattash R, Cilenti L, DuBois G, Lazebnik Y, Fernandes-Alnemri T and Alnemri ES . (2002). J. Biol. Chem., 277, 432–438.
Jia L, Macey MG, Yin Y, Newland AC and Kelsey SM . (1999). Blood, 93, 2353–2359.
Jia L, Patwari Y, Kelsey SM and Newland AC . (2001a). Biochem. Biophys. Res. Commun., 283, 1037–1045.
Jia L, Patwari Y, Srinivasula SM, Newland AC, Alnemri ES and Kelsey SM . (2001b). Oncogene, 20, 4817–4826.
Jia L, Srinivasula SM, Liu FT, Newland AC, Alnemri ES and Kelsey SM . (2001c). Blood, 98, 414–421.
Kabra NH, Kang C, Hsing LC, Zhang J and Winoto A . (2001). Proc. Natl. Acad. Sci. USA, 98, 6307–6312.
Liu FT, Kelsey SM, Newland AC and Jia L . (2002). Br. J. Haematol., 117, 333–342.
Liu X, Kim CN, Yang J, Jemmerson R and Wang X . (1996). Cell, 86, 147–157.
Li LY, Luo X and Wang X . (2001). Nature, 412, 95–99.
Liu Z, Sun C, Olejniczak ET, Meadows RP, Betz SF, Oost T, Herrmann J, Wu JC and Fesik SW . (2000). Nature, 408, 1004–1008.
Partheniou F, Kelsey SM, Srinivasula SM, Newland AC, Alnemri ES and Jia L . (2001). Biochem. Biophys. Res. Commun., 287, 181–189.
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH and Peter ME . (1998). EMBO J., 17, 1675–1687.
Srinivasula SM, Datta P, Fan XJ, Fernandes-Alnemri T, Huang Z and Alnemri ES . (2000). J. Biol. Chem., 275, 36152–36157.
Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, Lee RA, Fernandes-Alnemri T, Shi Y and Alnemri ES . (2001). Nature, 410, 112–116.
Suliman A, Lam A, Datta R and Srivastava RK . (2001). Oncogene, 20, 2122–2133.
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P,, Loeffler M and Kroemer G . (1999). Nature, 397, 441–446.
Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH and Reed JC . (2000). Clin. Cancer Res., 6, 1796–1803.
Terry NHA and White RA (2001). Methods Cell. Biol., 63, 355–374.
Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ and Vaux DL . (2000). Cell, 102, 43–53.
Zang DY, Goodwin RG, Loken MR, Bryant E and Deeg HJ . (2001). Blood, 98, 3058–3065.
Zhang XD, Zhang XY, Gray CP, Nguyen T and Hersey P . (2001). Cancer Res., 61, 7339–7348.
Acknowledgements
We acknowledge the Cancer Research UK Medical Oncology Unit for collection and storage of peripheral blood and bone marrow samples. This work was supported by grants from by the Leukaemia Research Fund (9946) to SMK and LJ and Research Advisor Committee of the Royal London Hospital (RAC389) to LJ.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jia, L., Patwari, Y., Kelsey, S. et al. Role of Smac in human leukaemic cell apoptosis and proliferation. Oncogene 22, 1589–1599 (2003). https://doi.org/10.1038/sj.onc.1206322
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1206322
Keywords
This article is cited by
-
Structure-based design, synthesis and anticancer effect of cyclic Smac–polyarginine peptides
Amino Acids (2018)
-
Non‐apoptotic functions of apoptosis‐regulatory proteins
EMBO reports (2012)
-
Effect of Smac on TRAIL-induced apoptosis of prostate cancer cell line PC-3 and the molecular mechanism
Journal of Huazhong University of Science and Technology [Medical Sciences] (2012)
-
Prognostic value of Smac expression in rectal cancer patients treated with neoadjuvant therapy
Medical Oncology (2012)
-
Sensitization of human bladder tumor cells to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis with a small molecule IAP antagonist
Apoptosis (2011)