Abstract
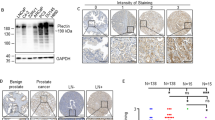
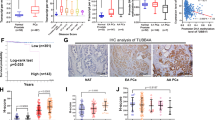
While studying Bim, a BH3-only proapoptotic protein, we identified an ∼36 kDa protein, which was abundantly expressed in all five strains of primary normal human prostate (NHP) epithelial cells but significantly reduced or lost in seven prostate cancer cell lines. The ∼36 kDa protein was subsequently identified as annexin II by proteomic approach and confirmed by Western blotting using an annexin II-specific antibody. Conventional and 2D SDS–PAGE, together with Western blotting, also revealed reduced or lost expression of annexin I in prostate cancer cells. Subcellular localization studies revealed that in NHP cells, annexin II was distributed both in the cytosol and underneath the plasma membrane, but not on the cell surface. Prostate cancer cells showed reduced levels as well as altered expression patterns of annexin II. Since annexins play important roles in maintaining Ca2+ homeostasis and regulating the cytoskeleton and cell motility, we hypothesized that the reduced or lost expression of annexin I/II might promote certain aggressive phenotypes of prostate cancer cells. In subsequent experiments, we indeed observed that restoration of annexin II expression inhibited the migration of the transfected prostate cancer cells without affecting cell proliferation or apoptosis. Hence, our results suggest that annexin II, and, likely, annexin I, may be endogenous suppressors of prostate cancer cell migration and their reduced or lost expression may contribute to prostate cancer development and progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Abbreviations
- 2D-PAGE:
-
two-dimensional polyacrylamide gel electrophoresis
- aa:
-
amino acids
- BrdU:
-
5-bromo-2′-deoxyuridine
- DAPI:
-
4′, 6-diamidino-2-phenylindole
- hrGFP:
-
humanized Renilla green fluorescence protein
- MALDI-TOF:
-
matrix-assisted laser desorption/ionization time of flight
- MS:
-
mass spectrometry
- NHP:
-
normal human prostate epithelial cells
- PSD:
-
postsource decay
References
Aarli A, Kristofferson EK, Jensen TS, Ulvestad E and Matre R . (1997). Am. J. Reprod. Immunol., 38, 313–319.
Ahn SH, Sawada H, Ro JY and Nicolson GL . (1997). Clin. Exp. Metastasis, 15, 151–156.
Balch C and Dedman JR . (1997). Exp. Cell Res., 237, 259–263.
Bianchi R, Garbuglia M, Verzini M, Giambanco I, Spreca A and Donata R . (1995). Biochem. Biophys. Res. Commun., 208, 910–918.
Chetcuti A, Margan SH, Russell P, Mann S, Millar D, Clark SJ, Rogers J, Handelsman DJ and Dong Q . (2001). Cancer Res., 61, 6331–6334.
Chiang Y, Davis RG and Vishwanatha JK . (1996). Biochim. Biophys. Acta, 1313, 295–301.
Chiang Y, Rizzino A, Sibenaller ZA, Wold MS and Vishwanatha JK . (1999). Mol. Cell. Biochem., 199, 139–147.
Chung CY and Erickson HP . (1994). J. Cell. Biol., 126, 539–548.
Cole SP, Pinkoski MJ, Bhardwaj G and Deeley RG . (1992). Br. J. Cancer, 65, 498–502.
Elkso E and Erikson RL . (1980). Cell, 21, 829–836.
Frohlich M, Motte P, Galvin K, Takahashi H, Wands J and Ozturk M . (1990). Mol. Cell. Biol., 10, 3216–3223.
Gupta S, Hussain T, Maclennan GT, Patel J and Mukhtar H . (2002). Proc. AACR, 43, A5.
Hajjar KA and Acharya SS . (2000). Ann. N. Y. Acad. Sci., 902, 265–271.
Hajjar KA, Jacovina AT and Chacko J . (1994). J. Biol. Chem., 269, 21191–21197.
Huang KS, Wallner BP, Mattaliano RJ, Tizard R, Burne C, Frey A, Hession C, McGray P, Sinclair LK and Chow EP . (1986). Cell, 46, 191–199.
Ikebuchi NW and Waisman DM . (1990). J. Biol. Chem., 265, 3392–3400.
Lee SW, Tomasetto C, Swisshelm C, Keyomarsi K and Sager R . (1992). Proc. Natl. Acad. Sci. USA, 89, 2504–2508.
Liu J-W, Chandra C, Tang S-H, Chopra D and Tang DG . (2002). Cancer Res., 62, 2976–2982.
Ma ASP, Bell DJ, Mittal AA and Harrison HH . (1994). J. Cell. Sci., 107, 1973–1984.
Ma ASP and Ozers LJ . (1996). Arch. Dermatol. Res., 288, 596–603.
Mai J, Waisman DM and Sloane BF . (2000). Biochim. Biophys. Acta, 1477, 215–230.
Menna C, Devlin RD, Reddy SV, Gazitt Y, Choi SJ and Roodman GD . (1999). J. Clin. Invest., 103, 1605–1613.
Morgan OR and Fernandez MP . (1997). Cell. Mol. Life Sci., 53, 508–511.
Navone NM, Olive M, Ozen M, Davis R, Tronsco P, Tu S-M, Johnston D, Pollack A, Pathak S, von Eschenbach AC and Logothetis CJ . (1997). Clin. Cancer Res., 3, 2493–2500.
Paweletz CP, Ornstein DK, Roth MJ, Bichsel VE, Gillespie JW, Calvert VS, Vocke CD, Hewitt SM, Duray PH, Herring J, Wang QH, Hu N, Linehan WM, Taylor PR, Liotta LA, Emmert-Buck MR and Petricoin III EF . (2000). Cancer Res., 60, 6293–6297.
Pencil SD and Toth M . (1998). Clin. Exp. Metastasis, 16, 113–121.
Pol A, Ortega D and Enrich C . (1997). Biochem. J., 327, 741–746.
Raynal P and Pollard HB . (1994). Biochim. Biophys. Acta, 1197, 63–93.
Reeves SA, Chavez-Kappel C, Davis R, Rosenblum M and Isreal MA . (1992). Cancer Res., 52, 6871–6876.
Rosenfeld J, Capdevielle J, Guillemot JC and Ferrara P . (1992). Anal. Biochem., 203, 173–179.
Solito E, de Coupade C, Canaider S, Goulding NJ and Perretti M . (2001). Br. J. Pharmacol., 133, 217–228.
Srivastava M, Bubendorf L, Srikantan V, Fossom L, Nolan L, Glasman M, Leighton X, Fehrle W, Pittaluga S, Raffeld M, Koivisto P, Willi N, Gasser TC, Kononen J, Sauter G, Kallioniemi OP, Srivastava S and Pollard HB . (2001). Proc. Natl. Acad. Sci. USA, 98, 4575–4580.
Takahashi S, Reddy SV, Chirgwin JM, Devlin R, Haipek C, Anderson J and Roodman GD . (1994). J. Biol. Chem., 269, 28696–28701.
Tang S, Bhatia B, Maldonado C, Yang P, Newman R, Liu J, Chandra D, Traag J, Klein RD, Fischer SM, Chopra D, Shen J, Zhau H, Chung LW and Tang DG . (2002). J. Biol. Chem., 277, 16189–16201.
Tang DG, Li L, Chopra D and Porter AT . (1998). Cancer Res., 58, 3466–3479.
Thiel C, Osborn M and Gerke V . (1992). J. Cell Sci., 103, 733–742.
Vishwanatha JK, Chiang Y, Kumber KD, Holingsworth MA and Pour PM . (1993). Carcinogenesis, 14, 2575–2579.
Zobiak N, Gerke V and Rescher U . (2001). FEBS Lett., 500, 137–140.
Zokas L and Glenny Jr JR . (1987). J. Cell Biol., 105, 2111–2121.
Acknowledgements
We thank C Conti for providing C4-2, C5, and MDA2b cells, D Chopra for NHP2 and NHP5 cells, and S Krishnan for assisting in MALDI-TOF analysis. This work was supported, in part, by the National Institute of Health Grants CA-90297 (DGT) and GM39338 (SSL), NIEHS Center Grant ES07784 (JJS, DGT, MDP, SSL), and MDACC institutional grants (DGT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, JW., Shen, JJ., Tanzillo-Swarts, A. et al. Annexin II expression is reduced or lost in prostate cancer cells and its re-expression inhibits prostate cancer cell migration. Oncogene 22, 1475–1485 (2003). https://doi.org/10.1038/sj.onc.1206196
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1206196
Keywords
This article is cited by
-
Promising effects of parasite-derived compounds on tumor regression: a systematic review of in vitro and in vivo studies
Environmental Science and Pollution Research (2022)
-
Plasma membrane integrity in health and disease: significance and therapeutic potential
Cell Discovery (2021)
-
Prognostic significance of annexin A2 and annexin A4 expression in patients with cervical cancer
BMC Cancer (2016)
-
Dynamic reciprocity: the role of annexin A2 in tissue integrity
Journal of Cell Communication and Signaling (2014)
-
Serum annexin A2 levels in acute brucellosis and brucellar spondylodiscitis
European Journal of Clinical Microbiology & Infectious Diseases (2014)