Abstract
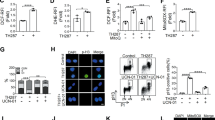
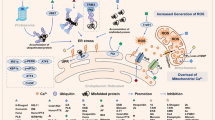
Aplidin™, a new antitumoural drug presently in phase II clinical trials, has shown both in vitro and in vivo activity against human cancer cells. Aplidin™ effectively inhibits cell viability by triggering a canonical apoptotic program resulting in alterations in cell morphology, caspase activation, and chromatin fragmentation. Pro-apoptotic concentrations of Aplidin™ induce early oxidative stress, which results in a rapid and persistent activation of both JNK and p38 MAPK and a biphasic activation of ERK. Inhibition of JNK and p38 MAPK blocks the apoptotic program induced by Aplidin™, demonstrating its central role in the integration of the cellular stress induced by the drug. JNK and p38 MAPK activation results in downstream cytochrome c release and activation of caspases -9 and -3 and PARP cleavage, demonstrating the mediation of the mitochondrial apoptotic pathway in this process. We also demonstrate that protein kinase C delta (PKC-δ) mediates the cytotoxic effect of Aplidin™ and that it is concomitantly processed and activated late in the apoptotic process by a caspase mediated mechanism. Remarkably, cells deficient in PKC-δ show enhanced survival upon drug treatment as compared to its wild type counterpart. PKC-δ thus appears as an important component necessary for full caspase cascade activation and execution of apoptosis, which most probably initiates a positive feedback loop further amplifying the apoptotic process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Anderson P . 1997 Microbiol. Mol. Biol. Rev. 61: 33–46
Anthoney A, Paz-Ares L, Twelves C, Cortes-Funes H, Kaye S, Pronk L, Celli N, Lopez-Lazaro L, Guzman C, Jimeno J . 2000 J. Clin. Oncol. 19: Suppl 734a
Armand JP, Ady-Vago N, Faivre S, Chieze S, Baudin E, Ribrag V, Lecot F, Iglesias L, Lopez-Lazaro L, Guzman C, Jimeno J, Ducreux M, Le Chevalier T, Raymond E . 2001 Proc. Am. Soc. Clin. Oncol. 20: (abstract 477)
Bacus SS, Gudkov AV, Lowe M, Lyass L, Yung Y, Komarov AP, Keyomarsi K, Yarden Y, Seger R . 2001 Oncogene 20: 147–155
Bharti A, Kraeft SK, Grounder M, Pandey P, Jin S, Yuam ZM, Lees-Miler SP, Weichselbaum R, Weaver D, Chen LB, Kufe D, Kharbanda S . 1998 Mol. Cell. Biol. 18: 6719–6728
Bowman A, Izquierdo MA, Jodrell D, Martinez M, Cicchella B, Jimeno J, Guzman C, Germa-Lluch J, Celli N, Smyth J . 2001 Proc. Am. Soc. Clin. Oncol. 20: (abstract 476)
Brenner B, Koppenhoefer U, Weinstock C, Linderkamp O, Lang F, Gulbins E . 1997 J. Biol. Chem. 272: 22173–22181
Broggini M, Marchini S, D'Incalci M, Taraboletti G, Giavazzi R, Faircloth G, Jimeno J . 2000 Proc. 11th NCI-EORTC-AACR Symposium on New Drugs in Cancer Therapy, (abstract 214)
Chen Y, Wang W, Kong AN, Tan TH . 1998 J. Biol. Chem. 273: 1769–1775
Chen Y, Wang X, Templeton D, Davis RJ, Tan TH . 1996a J. Biol. Chem. 271: 31929–31936
Chen YR, Meyer CF, Tan TH . 1996b J. Biol. Chem. 271: 631–634
Chen Z, Seimiya H, Naito M, Mashim T, Kizaki A, Dan S, Imauzumi M, Ichijo H, Miyazono K, Tsuruo T . 1999 Oncogene 18: 173–180
Ciruelos EM, Twelves C, Dominguez MJ, Anthony A, Castellanos D, Bezares S, Ruiz A, Lopez-Lazaro L, Jimeno J, Celli C, Cortes-Funes H, Paz-Ares L . 2002 Proc. Am. Soc. Clin. Oncol. 20: (abstract 442)
Cowan DB, Poutias DN, del Nido PJ, McGowan FXJ . 2000 Am. J. Physiol. Heart Circ. Physiol. 279: H619–H629
Crews CM, Collins JL, Lane WS, Snapper ML, Schreiber SL . 1994 J. Biol. Chem. 269: 15411–15414
Crews CM, Lane WS, Schreiber SL . 1996 Proc. Natl. Acad. Sci. USA 93: 4316–4319
Cross T, Griffiths G, Deacon E, Sallis R, Gough M, Watters D, Lod JM . 2000 Oncogene 19: 2331–2337
Ding W, Templeton DM . 2000 Toxicol. Appl. Pharmacol. 162: 93–99
English J, Pearson G, Wilsbacher J, Swantek J, Karandikar M, Xu S, Cobb MH . 1999 Exp. Cell Res. 253: 255–270
Erba E, Bassano L, Di Liberti G, Muradore I, Codegoni AM, Celli N, D'Incalci M . 1999 Proc. AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, (abstract 312)
Faircloth G, Grant W, Jimeno J, Meely K, Rinehart KL . 1998 Proc. Am. Assoc. Cancer Res. 39: (abstract 1551)
Faircloth G, Grant W, Nam S, Jimeno J, Manzanares I, Rinehart KL . 1999 Proc. Am. Assoc. Cancer Res. 40: (abstract 2612)
Figueroa-Masot XA, Hetman M, Higgins MJ, Kokot N, Xia Z . 2001 J. Neurosci. 21: 4657–4667
Grubb DR, Ly JD, Vaillant F, Johnson KL, Lawen A . 2001 Oncogene 20: 4085–4094
Gschwend M, Müller H, Kielbassa K, Zang R, Kittstein W, Rincke G, Marcks F . 1994 Biochem. Biophys. Res. Commun. 199: 93–98
Gschwendt M . 1999 Eur. J. Biochem. 259: 555–564
Hagemann C, Blank JL . 2001 Cell Signal 13: 863–875
Hay E, Lemonnier J, Fromigue O, Marie PJ . 2001 J. Biol. Chem. 276: 29028–29036
Herr I, Debatin KM . 2001 Blood 98: 2603–2614
Herr I, Wilhelm D, Bohler T, Angel P, Debatin KM . 1997 EMBO J. 16: 6200–6208
Hisamoto K, Ohmichi M, Kanda Y, Adachi K, Nishio Y, Hayakawa J, Mabuchi S, Takahashi K, Tasaka K, Miyamoto Y, Taniguchi N, Murata Y . 2001 J. Biol. Chem. 276: 47642–47649
Hofmann J . 2001 Rev. Physiol. Biochem. Pharmacol. 142: 1–96
Huang C, Ma WY, Li J, Dong Z . 1999 Cancer Res. 59: 3053–3058
Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Tagaki M, Matsumoto K, Miyazono K, Gotoh Y . 1997 Science 275: 90–94
Jacobson MD, Weil MRM . 1997 Cell 88: 347–354
Kang Y, Park JS, Kim S, Shin YJ, Kim W, Joo HJ, Chun JS, Kim HJ, Ha MJ . 2000 Mol. Cell. Biol. Res. Commun. 4: 181–187
Koji U, Mihoko S, Yuko N, Yasuyoshi T, Yoshimasa N, Toshihiko O . 1999 J. Biol. Chem. 274: 2234–2242
Kroemer G . 1997 Nat. Med. 3: 614–620
Krupinski J, Lopez E, Marti E, Ferrer I . 2000 Neurobiol. Dis. 7: 332–342
Kummer JL, Rao PK, Heidenreich KA . 1997 J. Biol. Chem. 272: 20490–20494
Leszczynski D . 1995 Oncol. Res. 7: 471–480
Lucas M, Sánchez-Margalet V . 1995 Gen. Pharmacol. 26: 881–887
Luo Y, Umegaki H, Wang X, Abe R, Roth GS . 1998 J. Biol. Chem. 273: 3756–3764
Marshall CJ . 1995 Cell 80: 179–185
Mauroun JA, Goel R, Stewart DJ, Tomiak E, Belanger K, Soulieres D, Charpentier D, Seymour L, Matthews S, Jimeno J, Guzman C . 2001 Proc. Am. Soc. Clin. Oncol. 20: (abstract 2082)
Miyamoto Y, Nakayama K, Imaki H, Hirose S, Jiang Y, Aba M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S, Nakayama KI . 2002 Nature 416: 865–869
Miyanishi K, Takayama T, Ohi M, Hayashi T, Nobuoka A, Nakajima T, Takimoto R, Kogawa K, Kato J, Sakamaki S, Niitsu Y . 2001 Gastroenterology 121: 865–874
Mizuno K, Noda K, Araki T, Imaoka T, Kobayashi Y, Akita Y, Shimonaka M, Kishi S, Ohno S . 1997 Eur. J. Biochem. 250: 7–18
Nuñez G, Benedict MA, Hu Y, Inohara N . 1998 Oncogene 17: 3237–3245
Osborn MT, Chambers TC . 1996 J. Biol. Chem. 271: 30950–30955
Persons DL, Yazlovitskaya EM, Cui W, Pelling JC . 1999 Clin. Cancer Res. 5: 1007–1014
Poot M, Teubert H, Rabinovitch PS, Kavanagh TJ . 1995 J. Cell Physiol. 163: 555–560
Powell CT, Brittis NJ, Stec D, Hug H, Heston WD, Fair WR . 1996 Cell Growth Differ. 7: 419–428
Rao L, White E . 1997 Curr. Opin. Genet. Dev. 7: 52–58
Reusch HP, Schaefer M, Plum C, Schultz G, Paul M . 2001 J. Biol. Chem. 276: 19540–19547
Reyland ME, Anderson SM, Matassa AA, Barzen KA, Quissell DO . 1999 J. Biol. Chem. 274: 19115–19123
Rinehart KL . 2000 Med. Res. Rev. 20: 1–27
Robinson MJ, Cobb MH . 1997 Curr. Opin. Cell Biol. 8: 180–186
Sambrook J, Fritsch EF, Maniatis T . 1989 Molecular Cloning: A Laboratory Manual New York: Cold Spring Harbor Laboratory Press
Sánchez A, Alvarez AM, Benito M, Fabregat I . 1996 J. Biol. Chem. 271: 7416–7422
Schaeffer HJ, Weber MJ . 1999 Mol. Cell Biol. 19: 2435–2444
Schnelldorfer T, Gansauge S, Gansauge F, Schlosser S, Beger HG, Nussler AK . 2000 Cancer 89: 1440–1447
Schwenger P, Bellosta P, Vietor I, Basilico C, Skolnik EY, Vilcek J . 1997 Proc. Natl. Acad. Sci. USA 94: 2869–2873
Seger R, Krebs EG . 1995 FASEB J. 9: 726–735
Seimiya H, Mashimo T, Toho M, Tsuruo T . 1997 J. Biol. Chem. 272: 4631–4636
Shao RG, Cao CX, Pommier Y . 1997 J. Biol. Chem. 272: 31321–31325
Shtil AA, Mandlekar S, Yu R, Walter RJ, Hagen K, Tan TH, Roninson IB, Kong AN . 1999 Oncogene 18: 377–384
Stempka L, Schnölzer M, Rincke G, Marks F, Gschwendt M . 1998 In ASBMB Symposium on Protein Kinase C and Cellular Function, (abstract 33)
Tewari M, Quan LT, O'Rouke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM . 1995 Cell 81: 801–809
Thompson CB . 1995 Science 267: 1456–1462
Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S . 1996 J. Biol. Chem. 271: 23512–23519
Whelan RD, Hosking LK, Townsend AJ, Cowan KH, Hill BT . 1989 Cancer Commun. 1: 359–365
Wyllie AH, Kerr JF, Currie AR . 1980 Int. Rev. Cytol. 68: 251–306
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME . 1995 Science 270: 1326–1331
Yu R, Jiao JJ, Duh JL, Tan TH, Kong AN . 1996a Cancer Res. 56: 2954–2959
Yu R, Shtil AA, Tan TH, Roninson IB, Kong AN . 1996b Cancer Lett. 107: 73–81
Yu W, Liao QY, Hantash FM, Sanders BG, Kline K . 2001 Cancer Res. 61: 6569–6576
Zhao X, Gschwend JE, Powell CT, Foster RG, Day KC, Day ML . 1997 J. Biol. Chem. 272: 22751–22757
Acknowledgements
We would like to thank Juan M López-Oliva for excellent technical assistance and members of the R+D Department of PharmaMar, S.A. We also thank Drs Simon Munt and José Antonio López-Martín for critical reading of the manuscript and fruitful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Fernández, L., Losada, A., Alcaide, V. et al. Aplidin™ induces the mitochondrial apoptotic pathway via oxidative stress-mediated JNK and p38 activation and protein kinase C δ. Oncogene 21, 7533–7544 (2002). https://doi.org/10.1038/sj.onc.1205972
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1205972
Keywords
This article is cited by
-
Therapeutic potential of marine peptides in cervical and ovarian cancers
Molecular and Cellular Biochemistry (2022)
-
Novel molecules as the emerging trends in cancer treatment: an update
Medical Oncology (2022)
-
Marine-derived pipeline anticancer natural products: a review of their pharmacotherapeutic potential and molecular mechanisms
Future Journal of Pharmaceutical Sciences (2021)
-
MAPK signaling pathway-targeted marine compounds in cancer therapy
Journal of Cancer Research and Clinical Oncology (2021)
-
HNGF6A Inhibits Oxidative Stress-Induced MC3T3-E1 Cell Apoptosis and Osteoblast Phenotype Inhibition by Targeting Circ_0001843/miR-214 Pathway
Calcified Tissue International (2020)