Abstract
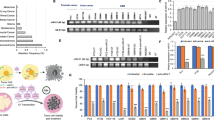
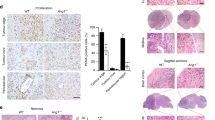
Matrix metalloproteinase 9 (MMP-9) is known to play a major role in cell migration and invasion in both physiological and pathological processes. Our previous work has shown that increased MMP-9 levels are associated with human glioma tumor progression. In this study, we evaluated the ability of an adenovirus containing a 528 bp cDNA sequence in antisense orientation to the 5′ end of the human MMP-9 gene (Ad-MMP-9AS) to inhibit the invasiveness and migratory capacity of the human glioblastoma cell line SBN19 in in vitro and in vivo models. Infection of glioma cells with Ad-MMP-9AS reduced MMP-9 enzyme activity by approximately 90% compared with mock- or Ad-CMV-infected cells. Migration and invasion of glioblastoma cells infected with Ad-MMP-9AS were significantly inhibited relative to Ad-CMV-infected controls in spheroid and Matrigel assays. Intracranial injections of SNB19 cells infected with Ad-MMP-9AS did not produce tumors in nude mice. However, injecting the Ad-MMP-9AS construct into subcutaneous U87MG tumors in nude mice caused regression of tumor growth. These results support the theory that adenoviral-mediated delivery of the MMP-9 gene in the antisense orientation has therapeutic potential for treating gliomas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Abbreviations
- ECM:
-
extracellular matrix
- MMP:
-
matrix metalloproteinase
- MT-MMP:
-
membrane-type MMP
- Ad:
-
adenovirus
- CMV:
-
cytomegalovirus
- MOI:
-
multiplicities of infection
- PFU:
-
plaque-forming units
- PMA:
-
phorbol myristate acetate
References
Arndt GM, Rank GH . 1997 Genome 40: 785–797
Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L . 2001 Mol. Biol. Cell 12: 863–879
Adachi Y, Lakka SS, Chandrasekar N, Yanamandra N, Gondi CS, Mohanam S, Dinh DH, Olivero WC, Gujrati M, Tamiya T, Ohmoto T, Kouraklis G, Aggarwal B, Rao JS . 2001 J. Biol. Chem. 276: 47171–47177
Baker AH, Zaltsman AB, George SJ, Newby AC . 1998 J. Clin. Invest. 101: 1478–1487
Baxter AD, Bird JB, Bannister RBRMDT, Watson RW, Owen DA, Montana JHJaJRC . 2000 Matrix Metalloproteinase Inhibitors in Cancer Therapy Clendeninn NJ and Appelt K. (ed.) Totowa, NJ: Humana Press, Inc. pp. 93–221
Brown PD . 1999 APMIS 107: 174–180
Brown PD . 2000 Expert. Opin. Investig. Drugs 9: 2167–2177
Chambers AF, Matrisian LM . 1997 J. Natl. Cancer Inst. 89: 1260–1270
Chintala SK, Sawaya R, Gokaslan ZL, Fuller G, Rao JS . 1996a Cancer Lett. 101: 107–114
Chintala SK, Sawaya R, Gokaslan ZL, Rao JS . 1996b Cancer Lett. 102: 57–63
Collier IE, Wilhelm SM, Eisen AZ, Marmer BL, Grant GA, Seltzer JL, Kronberger A, He CS, Bauer EA, Goldberg GI . 1988 J. Biol. Chem. 263: 6579–6587
Crabbe T, O'Connell JP, Smith BJ, Docherty AJ . 1994 Biochemistry 33: 14419–14425
Davies B, Brown PD, East N, Crimmin MJ, Balkwill FR . 1993a Cancer Res. 53: 2087–2091
Davies B, Waxman J, Wasan H, Abel P, Williams G, Krausz T, Neal D, Thomas D, Hanby A, Balkwill F . 1993b Cancer Res. 53: 5365–5369
Dubois B, Masure S, Hurtenbach U, Paemen L, Heremans H, van den OJ, Sciot R, Meinhardt T, Hammerling G, Opdenakker G, Arnold B . 1999 J. Clin. Invest. 104: 1507–1515
Eberhardt W, Huwiler A, Beck KF, Walpen S, Pfeilschifter J . 2000 J. Immunol. 165: 5788–5797
Folkman J . 1999 Nat. Biotechnol. 17: 749
Forsyth PA, Laing TD, Gibson AW, Rewcastle NB, Brasher P, Sutherland G, Johnston RN, Edwards DR . 1998 J. Neurooncol. 36: 21–29
Garbisa S, Pozzatti R, Muschel RJ, Saffiotti U, Ballin M, Goldfarb RH, Khoury G, Liotta LA . 1987 Cancer Res. 47: 1523–1528
Go Y, Chintala SK, Mohanam S, Gokaslan Z, Venkaiah B, Bjerkvig R, Oka K, Nicolson GL, Sawaya R, Rao JS . 1997 Clin. Exp. Metast. 15: 440–446
Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP . 1997 Eur. J. Cell. Biol. 74: 111–122
Graham FL, Prevec L . 1991 Methods in Molecular Biology. Murray EJ (ed.) Clifton, NJ: The Humana Press pp. 109–127
Houde M, de Bruyne G, Bracke M, Ingelman-Sundberg M, Skoglund G, Masure S, van Damme J, Opdenakker G . 1993 Int. J. Cancer 53: 395–400
Hua J, Muschel RJ . 1996 Cancer Res. 56: 5279–5284
Imai K, Yokohama Y, Nakanishi I, Ohuchi E, Fujii Y, Nakai N, Okada Y . 1995 J. Biol. Chem. 270: 6691–6697
Jones JL, Glynn P, Walker RA . 1999 J. Pathol. 189: 161–168
Kahari VM, Saarialho-Kere U . 1999 Ann. Med. 31: 34–45
Kin Y, Chintala SK, Go Y, Sawaya R, Mohanam S, Kyritsis AP, Rao JS . 2000 Int. J. Oncol. 17: 61–65
Kondraganti S, Mohanam S, Chintala SK, Kin Y, Jasti SL, Nirmala C, Lakka SS, Adachi Y, Kyritsis AP, Ali-Osman F, Sawaya R, Fuller GN, Rao JS . 2000 Cancer Res. 60: 6851–6855
Konduri SD, Tasiou A, Chandrasekar N, Nicolson GL, Rao JS . 2000 Clin. Exp. Metast. 18: 303–308
Koochekpour S, Merzak A, Pilkington GJ . 1995 Neurosci. Lett. 186: 53–56
Lakka SS, Jasti SL, Kyritsis AP, Yung WK, Ali-Osman F, Nicolson GL, Rao JS . 2000 Clin. Exp. Metast. 18: 245–252
Liotta LA, Steeg PS, Stetler-Stevenson WG . 1991 Cell 64: 327–336
Lozonschi L, Sunamura M, Kobari M, Egawa S, Ding L, Matsuno S . 1999 Cancer Res. 59: 1252–1258
Mohan PM, Lakka SS, Mohanam S, Kin Y, Sawaya R, Kyritsis AP, Nicolson GL, Rao JS . 1999 Clin. Exp. Metast. 17: 617–621
Nakada M, Nakamura H, Ikeda E, Fujimoto N, Yamashita J, Sato H, Seiki M, Okada Y . 1999 Am. J. Pathol. 154: 417–428
Nakajima M, Welch DR, Wynn DM, Tsuruo T, Nicolson GL . 1993 Cancer Res. 53: 5802–5807
Parks RJ, Bramson JL . 1999 Gene Ther. 6: 1349–1350
Price A, Shi Q, Morris D, Wilcox ME, Brasher PM, Rewcastle NB, Shalinsky D, Zou H, Appelt K, Johnston RN, Yong VW, Edwards D, Forsyth P . 1999 Clin. Cancer Res. 5: 845–854
Rao JS, Steck PA, Mohanam S, Stetler-Stevenson WG, Liotta LA, Sawaya R . 1993 Cancer Res. 53: 2208–2211
Rao JS, Yamamoto M, Mohaman S, Gokaslan ZL, Fuller GN, Stetler-Stevenson WG, Rao VH, Liotta LA, Nicolson GL, Sawaya RE . 1996 Clin. Exp. Metast. 14: 12–18
Rasmussen HS, McCann PP . 1997 Pharmacol. Ther. 75: 69–75
Sato H, Seiki M . 1993 Oncogene 8: 395–405
Scatena R . 2000 Expert. Opin. Investig. Drugs 9: 2159–2165
Seiki M . 1999 APMIS 107: 137–143
Shalinsky DR, Brekken J, Zou H, McDermott CD, Forsyth P, Edwards D, Margosiak S, Bender S, Truitt G, Wood A, Varki NM, Appelt K . 1999 Ann. NY Acad. Sci. 878: 236–270
Shingleton WD, Hodges DJ, Brick P, Cawston TE . 1996 Biochem. Cell. Biol. 74: 759–775
Stetler-Stevenson WG, Aznavoorian S, Liotta LA . 1993 Annu. Rev. Cell. Biol. 9: 541–573
Tonn JC, Kerkau S, Hanke A, Bouterfa H, Mueller JG, Wagner S, Vince GH, Roosen K . 1999 Int. J. Cancer 80: 764–772
Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z . 1998 Cell 93: 411–422
Wilhelm SM, Collier IE, Marmer BL, Eisen AZ, Grant GA, Goldberg GI . 1989 J. Biol. Chem. 264: 17213–17221
Yamamoto M, Mohanam S, Sawaya R, Fuller GN, Seiki M, Sato H, Gokaslan ZL, Liotta LA, Nicolson GL, Rao JS . 1996 Cancer Res. 56: 384–392
Yip D, Ahmad A, Karapetis CS, Hawkins CA, Harper PG . 1999 Invest. New Drugs 17: 387–399
Acknowledgements
We thank Karen Minter for preparing the manuscript and Christine Wogan for editorial review. Supported by NIH grants (CA76350 and CA75557; Jasti S Rao).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lakka, S., Rajan, M., Gondi, C. et al. Adenovirus-mediated expression of antisense MMP-9 in glioma cells inhibits tumor growth and invasion. Oncogene 21, 8011–8019 (2002). https://doi.org/10.1038/sj.onc.1205894
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1205894
Keywords
This article is cited by
-
Viral vector: potential therapeutic for glioblastoma multiforme
Cancer Gene Therapy (2020)
-
RUNX1 Regulates Migration, Invasion, and Angiogenesis via p38 MAPK Pathway in Human Glioblastoma
Cellular and Molecular Neurobiology (2017)
-
Systematic Review of Protein Biomarkers of Invasive Behavior in Glioblastoma
Molecular Neurobiology (2014)
-
Antisense MMP-9 RNA inhibits malignant glioma cell growth in vitro and in vivo
Neuroscience Bulletin (2013)
-
RRP22: a novel neural tumor suppressor for astrocytoma
Medical Oncology (2012)