Abstract
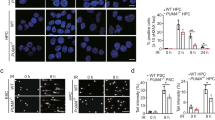
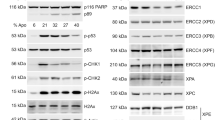
Molecular interactions among cell cycle and DNA repair proteins have been described, but the impact of many of these interactions on cell cycle control and DNA repair remains unclear. The cyclin-dependent kinase inhibitor, p21, is known to be involved in DNA damage-induced cell cycle arrest and blocking DNA replication and repair. Participation of p21 has been implicated in nucleotide excision repair. However, the role of p21 in the base excision repair (BER) pathway has not been thoroughly studied. In the present investigation, we treated isogenic mouse embryonic fibroblast (MEF) cell lines containing wild-type (MEF-polβ) or DNA polymerase β (polβ) gene-knockout (MEFpolβKO) with oxidative DNA-damaging agent, plumbagin, and examined its effect on p21 levels and BER activity. Plumbagin treatment caused a S-G2/M phase arrest and cell death of both MEF cell lines, induced p21 levels, and decreased p21-mediated long-patch (LP) BER by blocking DNA ligase activity in the polβ-dependent pathway and by blocking both FEN1 and DNA ligase activity in polβ-independent pathway. These findings suggest that plumbagin induced p21 levels play a regulatory role in cell cycle arrest, apoptosis, and polβ-dependent and -independent LP-BER pathways in MEF cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Abbreviations
- AP:
-
apurinic/apyrimidinic
- APE:
-
AP endonuclease
- bp:
-
base pair
- BER:
-
base excision repair
- cccDNA:
-
covalently closed circular DNA
- dRP:
-
2′-deoxyribose 5′-phosphate
- dRPase:
-
2′-deoxyribose 5′-phosphatase
- FEN1:
-
flap endonuclease 1
- LP:
-
long-patch
- MEF:
-
mouse embryonic fibroblast cell line
- PAGE:
-
polyacrylamide gel electrophoresis
- polβ:
-
DNA polymerase β
- polβKO:
-
polβ-gene knocked out
- PCNA:
-
proliferating cell nuclear antigen
- SP:
-
short-patch
- UDG:
-
uracil-DNA glycosylase
References
Agrawal S, Agarwal ML, Chatterjee-Kishore M, Stark GR, Chisolm GM . 2002 Mol. Cell. Biol. 22: 1981–1992
Ames BN . 1991 Jap. J. Cancer Res. 82: 1460–1461
Ando T, Kawabe T, Ohara H, Ducommun B, Itoh M, Okamoto T . 2001 J. Biol. Chem. 276: 42971–42977
Beckman KB, Ames BN . 1997 J. Biol. Chem. 272: 19633–19636
Bennett SE, Sung JS, Mosbaugh DW . 2001 J. Biol. Chem. 276: 42588–42600
Bjoras M, Luna L, Johnsen B, Hoff E, Haug T, Rognes T, Seeberg E . 1997 EMBO J. 16: 6314–6322
Bedford P, Fichtinger-Schepman AM, Shellard SA, Walker MC, Masters JR, Hill BT . 1998 Cancer Res. 48: 3019–3024
Bhattacharyya N, Chen HC, Comhair S, Erzurum SC, Banerjee S . 1999 DNA Cell Biol. 18: 549–554
Biade S, Sobol RW, Wilson SH, Matsumoto Y . 1998 J. Biol. Chem. 273: 898–902
Boulaire J, Fotedar A, Fotedar R . 2000 Pathol. Biol. (Paris) 48: 190–202
Brozmanova J, Dudas A, Henriques JA . 2001 Neoplasma 48: 85–93
Cadet J, Bourdat AG, D'Ham C, Duarte V, Gasparutto D, Romieu A, Ravanat JL . 2000 Mut. Res. 462: 121–128
Canitrot Y, Hoffmann JS, Calsou P, Hayakawa H, Salles B, Cazaux C . 2000 FASEB J. 14: 1765–1774
Cayrol C, Knibiehler M, Ducommun B . 1998 Oncogene 16: 311–320
Cooper MP, Balajee AS, Bohr VA . 1999 Mol. Biol. Cell. 10: 2119–2129
Dianov GL, Prasad R, Wilson SH, Bohr VA . 1999 J. Biol. Chem. 274: 13741–13743
Dimitriadis EK, Prasad R, Vaske MK, Chen L, Tomkinson AE, Lewis MS, Wilson SH . 1998 J. Biol. Chem. 273: 20540–20550
Dotto GP . 2000 Biochim. Biophys. Acta 1471: M43–M56
Dulic V, Stein GH, Far DF, Reed SI . 1999 Mol. Cell. Biol. 19: 205–215
Esposito F, Cuccovillo F, Russo L, Casella F, Russo T, Cimino F . 1998 Cell Death Differ. 5: 940–945
Fortini P, Pascucci B, Parlanti E, Sobol RW, Wilson SH, Dogliotti E . 1998 Biochemistry 37: 3575–3580
Fotedar R, Fitzgerald P, Rousselle T, Cannella D, Doree M, Messier H, Fotedar A . 1996 Oncogene 12: 2155–2164
Frosina G, Fortini P, Rossi O, Carrozzino F, Raspaglio G, Cox LS, Lane DP, Abbondandolo A, Dogliotti E . 1996 J. Biol. Chem. 271: 9573–9578
Hill BT, Scanlon KJ, Hansson J, Harstrick A, Pera M, Fichtinger-Schepman AM, Shellard SA . 1994 Eur. J. Cancer 30A: 832–837
Holmquist GP . 1998 Mutat. Res. 400: 59–68
Horton JK, Prasad R, Hou E, Wilson SH . 2000 J. Biol. Chem. 275: 2211–2218
Jaiswal AS, Narayan S . 2002 Mutation Res. 500: 17–30
Jamieson DJ, Rivers SL, Stephen DW . 1994 Microbiology 140: 3277–3283
Jonsson ZO, Hubscher U . 1997 Bioessays 19: 967–975
Kato T, Watanabe M, Ohta T . 1994 Mutagenesis 9: 245–251
Kelman Z . 1997 Oncogene 14: 629–640
Kim K, Biade S, Matsumoto Y . 1998 J. Biol. Chem. 273: 8842–8848
Klungland A, Lindahl T . 1997 EMBO J. 16: 3341–3348
Koberle B, Grimaldi KA, Sunters A, Hartley JA, Kelland LR, Masters JR . 1997 Int. J. Cancer 70: 551–555
Krokan HE, Standal R, Slupphaug G . 1997 Biochem J. 325: 1–16
Levin DS, McKenna AE, Motycka TA, Matsumoto Y, Tomkinson AE . 2000 Curr. Biol. 10: 919–922
Li R, Waga S, Hannon GJ, Beach D, Stillman B . 1994a Nature 371: 534–537
Li Y, Jenkins CW, Nichols MA, Xiong Y . 1994b Oncogene 9: 2261–2268
Li X, Li J, Harrington J, Lieber MR, Burgers PMJ . 1995 J. Biol. Chem. 270: 22109–22112
McDonald 3rd ER, Wu GS, Waldman T, El-Deiry WS . 1996 Cancer Res. 56: 2250–2255
Nasmyth K . 1996 Science 274: 1643–1645
Narayan S, Jaiswal AS . 1997 J. Biol. Chem. 272: 30619–30622
Narayan S, Jaiswal AS, Multani AS, Pathak S . 2001 Br. J. Cancer 85: 898–901
Pan ZQ, Reardon JT, Li L, Flores-Rozas H, Legerski R, Sancar A, Hurwitz J . 1995 J. Biol. Chem. 270: 22008–22016
Prasad R, Dianov GL, Bohr VA, Wilson SH . 2000 J. Biol. Chem. 275: 4460–4466
Prasad R, Lavrik OI, Kim SJ, Kedar P, Yang XP, Vande Berg BJ, Wilson SH . 2001 J. Biol. Chem. 276: 32411–32414
Prieto-Alamo MJ, Abril N, Pueyo C . 1993 Carcinogenesis 14: 237–244
Prosperi E . 1997 Prog. Cell Cycle Res. 3: 193–210
Rousseau D, Cannella D, Boulaire J, Fitzgerald P, Fotedar A, Fotedar R . 1999 Oncogene 18: 4313–4325
Sark MW, Timmer-Bosscha H, Meijer C, Uges DR, Sluiter WJ, Peters WH, Mulder NH, de Vries EG . 1995 Br. J. Cancer 71: 684–690
Seeberg E, Eide L, Bjoras M . 1995 Trends Biochem. Sci. 20: 391–397
Sheikh MS, Chen YQ, Smith ML, Fornace Jr AJ . 1997 Oncogene 14: 1875–1882
Shivji MK, Ferrari E, Ball K, Hubscher U, Wood RD . 1998 Oncogene 17: 2827–2838
Shivji MK, Grey SJ, Strausfeld UP, Wood RD, Blow JJ . 1994 Curr. Biol. 4: 1062–1068
Shortle D, Botstein D . 1983 Methods Enzymol. 100: 457–468
Sobol RW, Horton JK, Kuhn R, Gu H, Singhal RK, Prasad R, Rajewsky K, Wilson SH . 1996 Nature 379: 183–186
Sobol RW, Prasad R, Evenski A, Baker A, Yang XP, Horton JK, Wilson SH . 2000 Nature 405: 807–810
Srivastava DK, Berg BJ, Prasad R, Molina JT, Beard WA, Tomkinson AE, Wilson SH . 1998 J. Biol. Chem. 273: 21203–21209
Srivastava DK, Husain I, Arteaga CL, Wilson SH . 1999 Carcinogenesis 20: 1049–1054
Stivala LA, Riva F, Cazzalini O, Savio M, Prosperi E . 2001 Oncogene 20: 563–570
Sugie S, Okamoto K, Rahman KM, Tanaka T, Kawai K, Yamahara J, Mori H . 1998 Cancer Lett. 127: 177–183
Taylor WR, Stark GR . 2001 Oncogene 20: 1803–1815
Tom S, Ranalli TA, Podust VN, Bambara RA . 2001 J. Biol. Chem. 276: 48781–48789
Vindelov LL, Christensen IJ . 1990 Cytometry 11: 753–770
Waga S, Stillman B . 1998 Mol. Cell. Biol. 18: 4177–4187
Wang L, Patel U, Ghosh L, Banerjee S . 1992 Cancer Res. 52: 4824–4827
Warbrick E . 2000 Bioessays 22: 997–1006
Wilson SH . 1998 Mutat. Res. 407: 203–215
Acknowledgements
We would like to thank Drs Rajendra Prasad and Samuel H Wilson from NIEHS, Research Triangle Park, NC, for providing the neutralizing anti-polβ antibody and the mouse embryonic fibroblast (MEF) cell lines with wild-type and polβ-gene-knockout clones; Dr Arun Fotedar from the Cellular and Molecular Biology Program, Sidney Kimmel Cancer Center (San Diego, CA, USA), for providing us the wild-type and mutant GST-p21 protein overexpression vectors. We thank Jessica A Salinas for technical assistance and Nirupama Gupta for editorial comments. The FACS analysis was performed in the FCC Laboratory of the ICBR of the University of Florida. These studies were supported in part by the grants awarded to S Narayan from the National Cancer Institute, NIH (CA77721); the 2001-Research Opportunity Fund by the University of Florida (Gainesville, FL, USA); and the 2001-Ralph E Powe Junior Faculty Enhancement Award by the Oak Ridge Associated Universities (Oak Ridge, TN, USA). The work of LB Bloom was supported by NSF grant MCB-9722356.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaiswal, A., Bloom, L. & Narayan, S. Long-patch base excision repair of apurinic/apyrimidinic site DNA is decreased in mouse embryonic fibroblast cell lines treated with plumbagin: involvement of cyclin-dependent kinase inhibitor p21Waf-1/Cip-1. Oncogene 21, 5912–5922 (2002). https://doi.org/10.1038/sj.onc.1205789
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1205789
Keywords
This article is cited by
-
The ruthenium(II)–arene compound RAPTA-C induces apoptosis in EAC cells through mitochondrial and p53–JNK pathways
JBIC Journal of Biological Inorganic Chemistry (2008)
-
Cigarette smoke condensate-induced level of adenomatous polyposis coli blocks long-patch base excision repair in breast epithelial cells
Oncogene (2007)
-
Potential of radioiodinated anti cancer compounds of natural origin for cancer therapy
Journal of Radioanalytical and Nuclear Chemistry (2007)
-
Human 8-oxoguanine DNA glycosylase increases resistance to hyperoxic cytotoxicity in lung epithelial cells and involvement with altered MAPK activity
Cell Death & Differentiation (2006)
-
Reduced levels of the adenomatous polyposis coli (APC) protein are associated with ceramide-induced apoptosis of colon cancer cells
Journal of Cancer Research and Clinical Oncology (2004)