Abstract
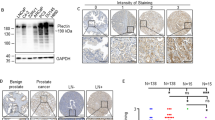
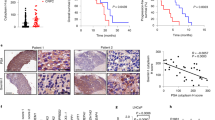
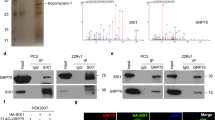
Prostate cancer is one of the most common malignant tumors with increasing incidence rates in the aging male. Since locally advanced or metastatic prostate tumors are essentially incurable, identification of new target molecules and treatment strategies is of critical importance. Fibroblast growth factor-2 (FGF-2) acts as potent mitogen which is upregulated in prostate cancers modulating cancer cell proliferation and development of an invasive phenotype. Normally it is tightly bound to the extracellular matrix that quenches its biological activity. The FGF-binding proteins (FGF-BP, HBp17) is a secreted protein which is able to mobilize and activate FGF-2 from the extracellular matrix. Here we show that FGF-BP is highly expressed in prostate tumor cells. To study the functional role of FGF-BP, we use a ribozyme-targeting approach to selectively deplete FGF-BP in prostate cancer cells achieving a more than 50% reduction of FGF-BP mRNA and protein levels in two mass-transfected cell lines. FGF-BP depletion reduces proliferation of the cells in vitro without changes in cell cycle distribution or apoptosis. Using cDNA microarrays, Northern blotting and RT–PCR, we show a complex pattern of changes in the gene expression profiles upon FGF-BP depletion. Most strikingly, ribozyme-mediated reduction of FGF-BP levels completely abolishes the ability of the highly metastatic PC-3 prostate carcinoma cells to grow tumors in an athymic nude mouse in vivo model which is far beyond the effects of FGF-BP ribozyme targeting observed previously in cells from other tumors in the same model. Taken together, our study identifies FGF-BP as a potential rate-limiting factor for prostate cancer growth and, due to its restricted expression pattern in adults, a potentially attractive target for prostate cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Abbreviations
- ECM:
-
extracellular matrix
- EGF:
-
epithelial growth factor
- ELISA:
-
enzyme-linked immunosorbent assay
- ERBIN:
-
HER-2 interacting protein
- FACS:
-
fluorescence-activated cell sorting
- FCS:
-
fetal calf serum
- FGF:
-
fibroblast growth factor
- FGF-BP:
-
fibroblast growth factor-binding protein
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- GST:
-
glutathione S-transferase
- HRP:
-
horseradish peroxidase
- IL-8:
-
interleukin-8
- PLAB:
-
placental bone morphogenetic protein
- Rz:
-
ribozyme
- SCC:
-
squamous cell carcinoma
- TPA:
-
12-O-tetradecanoylphorbol-13-acetate
- UCHL-1/PGP 9.5:
-
ubiquitin carboxy-terminal esterase
References
Aigner A, Butscheid M, Kunkel P, Krause E, Lamszus K, Wellstein A, Czubayko F . 2001 Int. J. Cancer 92: 510–517
Aigner A, Hsieh SS, Malerczyk C, Czubayko F . 2000a Toxiology 144: 221–228
Aigner A, Malerczyk C, Houghtling R, Wellstein A . 2000b Growth Factors 18: 51–62
Aigner A, Ray PE, Czubayko F, Wellstein A . 2002 Histochem. Cell Biol. 117: 1–11
Aumuller G, Renneberg H, Leonhardt M, Lilja H, Abrahamsson PA . 1999 Prostate 38: 261–267
Begun FP, Story MT, Hopp KA, Shapiro E, Lawson RK . 1995 J. Urol. 153: 839–843
Bokhoven van A, Varella-Garcia M, Korch C, Hessels D, Miller GJ . 2001 The Prostate 47: 36–51
Borg JP, Marchetto S, Le Bivic A, Ollendorff V, Jaulin-Bastard F, Saito H, Fournier E, Adelaide J, Margolis B, Birnbaum D . 2000 Nat. Cell. Biol. 2: 407–414
Chackal-Roy M, Niemeyer C, Moore M, Zetter BR . 1989 J. Clin. Invest. 84: 43–50
Chandler LA, Sosnowski BA, Greenlees L, Aukerman SL, Baird A, Pierce GF . 1999 Int. J. Cancer 81: 451–458
Czubayko F, Liaudet-Coopman EDE, Aigner A, Tuveson AT, Berchem G, Wellstein A . 1997 Nat. Med. 3: 1137–1140
Czubayko F, Smith RV, Chung HC, Wellstein A . 1994 J. Biol. Chem. 269: 28243–28248
Darzynkiewicz Z, Bruno S, Del Bino G, Gorczyca W, Hotz MA, Lassota P, Traganos F . 1992 Cytometry 13: 795–808
Dow JK, deVere White RW . 2000 Urology 55: 800–806
Dow JK, Thompson ID, Chaudhuri G . 1997 J. Urol. 157: 221 (abstract)
Giri D, Ropiquet F, Ittmann M . 1999a Clin. Cancer Res. 5: 1063–1071
Giri D, Ropiquet F, Ittmann M . 1999b J. Cell. Physiol. 180: 53–60
Gospodarowicz D, Ferrara N, Schweigerer L, Neufeld G . 1987 Endocr. Rev. 8: 95–114
Harris VK, Kagan BL, Ray R, Coticchia CM, Liaudet-Coopman ED, Wellstein A, Riegel AT . 2001 Oncogene 20: 1730–1738
Harris VK, Liaudet-Coopman EDE, Boyle BJ, Wellstein A, Riegel AT . 1998 J. Biol. Chem. 273: 19130–19139
Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD, Yano S, Bar-Eli M, Radinsky R, Pettaway CA, Dinney CP . 2000 Clin. Cancer Res. 6: 2104–2119
Ittman M, Mansukhani A . 1997 J. Urol. 157: 351–356
Kim SJ, Uehara H, Karashima T, McCarty M, Shih N, Fidler IJ . 2001 Neoplasia 3: 33–42
Kurtz A, Wang HL, Darwiche N, Harris V, Wellstein A . 1997 Oncogene 14: 2671–2681
La Rocca RV, Danesi R, Cooper MR, Jamis-Dow CA, Ewing MW, Linehan WM, Myers CE . 1991 J. Urol. 145: 393–398
Lametsch R, Rasmussen JT, Johnsen LB, Purup S, Sejrsen K, Petersen TE, Heegaard CW . 2000 J. Biol. Chem. 275: 19469–19474
Leung HY, Dickson C, Robson CN, Neal DE . 1996 Oncogene 12: 1833–1835
Liaudet-Coopman EDE, Berchem GJ, Wellstein A . 1997 Clin. Cancer Res. 3: 179–184
Liaudet-Coopman EDE, Schulte AM, Cardillo M, Wellstein A . 1996 Biochem. Biophys. Res. Commun. 229: 930–937
Liaudet-Coopman EDE, Wellstein A . 1996 J. Biol. Chem. 271: 21303–21308
Lieberman R, Nelson WG, Sakr WA, Meyskens Jr FL, Wilding G, Partin AW, Lee JJ, Lippman SM . 2001 Urology 57: 4–27
Martin R, Fraile B, Peinado F, Arenas MI, Elices M, Alonso L, Paniagua R, Martin JJ, Santamaria L . 2000 J. Histochem. Cytochem. 48: 1121–1130
Mehta PB, Robson CN, Neal DE, Leung HY . 2000 J. Urol. 164: 2151–2155
Middleman MN, Lush RM, Sartor O, Reed E, Figg WD . 1996 Cancer Treat. Rev. 22: 105–118
Mori H, Maki M, Oishi K, Jaye M, Igarashi K, Yoshida O, Hatanaka M . 1990 Prostate 16: 71–80
Nakamoto T, Chang CS, Li AK, Chodak GW . 1992 Cancer Res. 52: 571–577
Nishi N, Matuo Y, Kunitomi K, Takenaka I, Usami M, Kotake T, Wada F . 1988 Prostate 13: 39–48
Okamoto T, Tanaka Y, Kan M, Sakamoto A, Takada K, Sato JD . 1996 In Vitro Cell Dev. Biol. Anim. 32: 69–71
Ozen M, Giri D, Ropiquet F, Masukhani A, Ittmann M . 2001 J. Natl. Cancer Inst. 93: 1783–1790
Ropiquet F, Giri D, Kwabi-Addo B, Mansukhani A, Ittmann M . 2000 Cancer Res. 60: 4245–4250
Ropiquet F, Giri D, Lamb DJ, Ittmann M . 1999 J. Urol. 162: 595–599
Shain SA, Saric T, Ke LD, Nannen D, Yoas S . 1996 Cell Growth Differ. 7: 573–586
Stöppler H, Malerczyk C, Block K, Aigner A, Czubayko F . 2001 Oncogene 20: 7430–7436
Story MT, Livingston B, Baeten L, Swartz SJ, Jacobs SC, Begun FP, Lawson RK . 1989 Prostate 15: 355–365
Thomas R, True LD, Lange PH, Vessella RL . 2001 Int. J. Cancer 93: 47–52
Webber MM, Bello D, Quader S . 1997 The Prostate 30: 58–64
Wellstein A, Czubayko F . 1996 Breast Cancer Res. Treat. 38: 109–119
Wellstein A, Lupu R, Zugmaier G, Flamm SL, Cheville AL, Delli Bovi P, Basilico c, Lippman ME, Kern FG . 1990 Cell Growth Differ. 1: 63–71
Wu D, Kan M, Sato GH, Okamoto T, Sato JD . 1991 J. Biol. Chem. 266: 16778–16785
Yagoda A, Petrylak D . 1993 Cancer 71: 1098–1109
Yan G, Fukabori Y, McBride G, Nikolaropolous S, McKeehan WL . 1993 Mol. Cell. Biol. 13: 4513–4522
Acknowledgements
We are grateful to Andrea Wüstenhagen, Barbara Siegel, Helga Radler and Daniela Wagner for expert technical assistance, to Kirsten Frank for skilful handling of the animals and to Mary-Lou Zuzarte for expert help with cell cycle analysis. This work was supported by grants from the A und E Kulemann-Stiftung and by the Forschungspool of the Philipps-University to A Aigner, and the Deutsche Akademie der Naturforscher Leopoldina (fellowship grant to H Renneberg).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aigner, A., Renneberg, H., Bojunga, J. et al. Ribozyme-targeting of a secreted FGF-binding protein (FGF-BP) inhibits proliferation of prostate cancer cells in vitro and in vivo. Oncogene 21, 5733–5742 (2002). https://doi.org/10.1038/sj.onc.1205560
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1205560
Keywords
This article is cited by
-
Experimental in vitro, ex vivo and in vivo models in prostate cancer research
Nature Reviews Urology (2023)
-
A comparative study of clinicopathological significance, FGFBP1, and WISP-2 expression between squamous cell/adenosquamous carcinomas and adenocarcinoma of the gallbladder
International Journal of Clinical Oncology (2014)
-
Anti-tumor effects of fibroblast growth factor-binding protein (FGF-BP) knockdown in colon carcinoma
Molecular Cancer (2011)
-
RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo
Gene Therapy (2005)
-
The fibroblast growth factor binding protein is a novel interaction partner of FGF-7, FGF-10 and FGF-22 and regulates FGF activity: implications for epithelial repair
Oncogene (2005)