Abstract
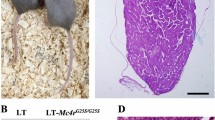
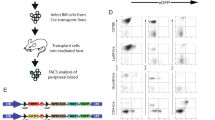
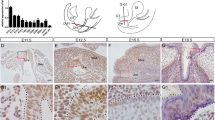
We previously demonstrated expression of the HST-1/FGF-4 gene in the testis of normal adult animals, which suggests its possible role in spermatogenesis. For an understanding of its functional significance in the testis, conditional transgene expression was used. Precise genetic switches can be efficiently generated in a straightforward manner using adenovirus-carrying Cre recombinase, which means our new strategies promise to contribute substantially to a better and prompt understanding of the functions of genes in vivo by controlling the expression of any gene to any organ at any desired time. Our new method demonstrated for the first time that the specific gain of function of the HST-1/FGF-4 gene in the testis resulted in markedly enhanced spermatogenesis. To further investigate the function and therapeutic potency of HST-1/FGF-4, transgenic mice with enhanced HST-1/FGF-4 expression in the testis were exposed to adriamycin (ADR), an anticancer drug causing severe testicular toxicity. Degree of damage to spermatogenesis was assessed by sperm count, testicular weight, histology, and DNA ploidy. Induced expression of HST-1/FGF-4 markedly enhanced the recovery of ADR-induced testicular damage. Furthermore, adenoviruses carrying the HST-1/FGF-4 gene ameliorated testicular toxicity of ADR. These results with new adenovirus-mediated Cre/lox conditional mice indicated that HST-1/FGF-4 could be an important factor for spermatogenesis, presenting a new paradigm to treat impaired fertility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Avarbock MR, Brinster CJ, Brinster RL . 1996 Nat. Med. 2: 693–696
Cancilla B, Risbridger GP . 1998 Biol. Reprod. 58: 1138–1145
Ford WC . 2001 Lancet 357: 1223–1224
Griswold MD . 1988 Int. Rev. Cytol. 110: 133–156
Griswold MD, Morales C, Sylvester SR . 1988 Oxf. Rev. Reprod. Biol. 10: 124–161
Grell RF, Oakberg EF, Generoso EE . 1980 Proc. Natl. Acad. Sci. USA 77: 6720–6723
Haimovitz-Friedman A, Balaban N, McLoughlin M, Ehleiter D, Michaeli J, Vlodavsky I, Fuks Z . 1994 Cancer Res. 54: 2591–2597
Hall SJ, Bar-Chama N, Ta S, Gordon JW . 2000 Hum. Gene Ther. 11: 1705–1712
Hellstrom WJ, Tesluk H, Deitch AD, de Vere White RW . 1990 Urology 35: 321–326
Hogan B, Constantini F, Lacy E . 1986 Manipulating the mouse embryo. A Laboratory Manual Cold Spring Harbor Laboratory
Ishii Y, Fukuda K, Saiga H, Matsushita S, Yasugi S . 1997 Dev. Growth Differ. 39: 643–653
Ivanova M, Mollova M, Ivanova-Kicheva MG, Petrov M, Djarkova T, Somlev B . 1999 Theriogenology 1: 163–170
Kanegae Y, Makimura M, Saito I . 1994 Jpn. J. Med. Sci. Biol. 47: 157–166
Kanegae Y, Lee G, Sato Y, Tanaka M, Nakai M, Sakaki T, Sugano S, Saito I . 1995 Nucleic Acids Res. 23: 3816–3821
Konishi H, Ochiya T, Sakamoto H, Tsukamoto M, Saito I, Muto T, Sugimura T, Terada M . 1995 J. Clin. Invest. 96: 1125–1130
Konishi H, Ochiya T, Yasuda Y, Sakamoto H, Muto T, Sugimura T, Terada M . 1996 Oncogene 13: 9–19
Le Magueresse-Battistoni B, Wolff J, Morera AM, Benahmed M . 1994 Endocrinol. 135: 2404–2411
Matsui H, Toyoda K, Shinoda K, Okamiya H, Furukawa F, Kawanishi T, Takahashi M . 1993 Eisei Shikenjo Hokoku 111: 39–46
Miho Y, Kouroku Y, Fujita E, Mukasa T, Urase K, Kasahara T, Isoai A, Momoi M, Momoi T . 1999 Cell Death Differ. 6: 463–470
Niswander L, Martin GR . 1992 Development 114: 755–768
Niswander L, Martin GR . 1993 Nature 361: 68–71
Niswander L, Jeffrey S, Martin GR, Tickle C . 1994 Nature 371: 609–612
Oakberg EF, Huckins C . 1976 Stem Cell of Renewing Cell Populations Cairnie AB, Lala P and Osmond DG (ed) Academic Press, New York pp 287–302
Ochiya T, Sakamoto H, Tsukamoto M, Sugimura T, Terada M . 1995 J. Cell Biol. 130: 997–1003
Ogawa T, Dobrinski I, Avarbock MR, Brinster RL . 2000 Nat. Med. 6: 29–34
Orth JM, Boehm R . 1990 Anat. Rec. 226: 320–327
Russo A, Levis AG . 1992 Environ. Mol. Mutagen. 19: 125–131
Sakamoto H, Mori M, Taira M, Yoshida T, Matsukawa S, Shimizu K, Sekiguchi M, Terada M, Sugimura T . 1986 Proc. Natl. Acad. Sci. USA 83: 3997–4001
Sakamoto H, Ochiya T, Sato Y, Tsukamoto M, Konishi H, Saito I, Sugimura T, Terada M . 1994 Proc. Natl. Acad. Sci. USA 91: 12368–12372
Sauer B . 1998 Methods 14: 381–392
Sharpe RM . 1994 Knobil E and Neil JD. (eds) Physiology of reproduction 6th edn Vol. 1: New York: Raven Press pp 1363–1434
Shinoda K, Mitsumori K, Yasuhara K, Uneyama C, Onodera H, Hirose M, Uehara M . 1999 Arch. Toxicol. 73: 274–281
Simpkins H, Pearlman LF, Thompson LM . 1984 Cancer Res. 44: 613–618
Simpkins H, Pearlman LF . 1984 Biochim. Biophys. Acta. 783: 293–300
Sjoblom T, West A, Lahdetie J . 1998 Environ. Mol. Mutagen. 31: 133–148
Steger K, Tetens F, Seitz J, Grothe C, Bergman M . 1998 Histochem. Cell Biol. 110: 57–62
Strohmeyer T, Peter S, Hartmann M, Munemitsu S, Ackermann R, Ullrich A, Slamon DJ . 1991 Cancer Res. 51: 1811–1816
Suzuki HR, Sakamoto H, Yoshida T, Sugimura T, Terada M, Solursh M . 1992 Dev. Biol. 150: 219–222
Takahama Y, Ochiya T, Tanooka H, Yamamoto H, Sakamoto H, Nakano H, Terada M . 1999 Oncogene 18: 5943–5947
Van Dissel-Emiliani FM, De Boer-Brouwer M, De Rooij DG . 1996 Endocrinol. 137: 647–654
Wagle A, Singh JP . 2000 J. Pharmacol. Exp. Ther. 295: 889–895
Werdien D, Peiler G, Ryffel GU . 2001 Nucleic Acids Res. 29: E53–
Wilkinson DG . 1992 In situ hybridization: A practical approach IRL Press: London
Yamamoto H, Ochiya T, Takahama Y, Ishii Y, Osumi N, Sakamoto H, Terada M . 2000 Oncogene 19: 3805–3810
Yoshida T, Ishimaru K, Sakamoto H, Yokota J, Hirohashi S, Igarashi K, Sudo K, Terada M . 1994 Cancer Lett. 15: 261–268
Yoshida T, Miyagawa K, Sakamoto H, Sugimura T, Terada M . 1991 Methods Enzymol. 198: 124–138
Yoshida T, Tsutsumi M, Sakamoto H, Miyagawa K, Teshima S, Sugimura T, Terada M . 1988 Biochem. Biophys. Res. Commun. 155: 1324–1329
Acknowledgements
We gratefully thank Dr Junichi Miyazaki (Osaka University, Japan) for his kind gift of CAG promoter. We thank Ms Maki Abe and Ms Masako Hosoda for their excellent technical work. This work was supported in part by a Grant-in-Aid for the Second-Term Comprehensive 10-Year Strategy for Cancer Control, Health Science Research Grants for the Research on Human Genome and Gene Therapy from the Ministry of Health, Labor and Welfare of Japan, and a Grant-in-Aid by Japan Owner's Association.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamamoto, H., Ochiya, T., Tamamushi, S. et al. HST-1/FGF-4 gene activation induces spermatogenesis and prevents adriamycin-induced testicular toxicity. Oncogene 21, 899–908 (2002). https://doi.org/10.1038/sj.onc.1205135
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1205135
Keywords
This article is cited by
-
Dysregulation of Notch-FGF signaling axis in germ cells results in cystic dilation of the rete testis in mice
Journal of Cell Communication and Signaling (2022)
-
Homology modeling and virtual screening studies of FGF-7 protein—a structure-based approach to design new molecules against tumor angiogenesis
Journal of Chemical Biology (2016)