Abstract
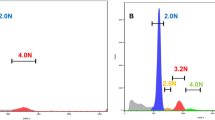
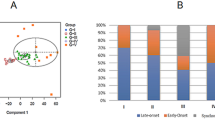
Colorectal cancer has been described in terms of genetic instability selectively affecting either microsatellite sequences (MIN) or chromosome number and structure (CIN). A subgroup with apparently stable, near-diploid chromosomes and stable microsatellites (MACS) also exists. These distinctions are important, partly because of their value in highlighting different pathways of carcinogenesis, and partly because of their direct relevance to prognosis. Study of early-onset cancer has often proved a fruitful resource for the identification of the nature and function of cancer susceptibility genes. In a study of colorectal cancer with stable microsatellite DNA, we describe 22 early-onset tumours (mean age=33), compared with 16 late-onset tumours (mean age=68). Both groups contained carcinomas with the MACS phenotype, characterized by near diploid DNA content, as defined by flow cytometry, and minimal chromosome arm deletion or amplification (six or less events per genome), determined by comparative genomic hybridization (CGH). Minimal chromosome imbalance correlated strongly with diploid DNA content (P<0.001). The proportion of MACS cancers was significantly greater in early-onset as compared to late-onset tumours (64 vs 13%, P=0.005). Of the chromosome arm imbalances commonly observed in late-onset tumours, only 18q− was observed more than twice amongst the 14 early-onset MACS tumours. Seventy-nine per cent of these MACS tumours were located in the distal colon, and 69% were at advanced clinico-pathological stages (with lymph node or distant metastasis). A positive family history of colorectal or other cancers was elicited in seven patients in the MACS early-onset group, and one additional patient in this group had a metachronous ovarian cancer. The results suggest that MACS cancer may have a genetic basis different from either MIN or CIN, and further studies of these cancers may lead to discovery of new mechanisms of colorectal carcinogenesis and cancer susceptibility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Aaltonen LA, Peltomaki P, Leach FS, Sistonen P, Pylkkanen L, Mecklin JP, Jarvinen H, Powell SM, Jen J, Hamilton SR, Petersen GM, Kinzler KW, Vogelstein B, de la Chapelle A . 1993 Science 260: 812–816
Bubb VJ, Curtis LJ, Cunningham C, Dunlop MG, Carothers AD, Morris RG, White S, Bird CC, Wyllie AH . 1996 Oncogene 12: 2641–2649
Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B . 1998 Nature 392: 300–303
Carder P, Wyllie AH, Purdie CA, Morris RG, White S, Piris J, Bird CC . 1993 Oncogene 8: 1397–1401
Chan TL, Yuen ST, Chung LP, Ho JW, Kwan KY, Chan AS, Ho JC, Leung SY, Wyllie AH . 1999 J. Natl. Cancer Inst. 91: 1221–1226
Clarke AR, Gledhill S, Hooper ML, Bird CC, Wyllie AH . 1994 Oncogene 9: 1767–1773
Cottu PH, Muzeau F, Estreicher A, Flejou JF, Iggo R, Thomas G, Hamelin R . 1996 Oncogene 13: 2727–2730
Curtis LJ, Georgiades IB, White S, Bird CC, Harrison DJ, Wyllie AH . 2000 J. Pathol. 192: 440–445
De Angelis PM, Clausen OP, Schjolberg A, Stokke T . 1999 Br. J. Cancer 80: 526–535
Farrington SM, Lin-Goerke J, Ling J, Wang Y, Burczak JD, Robbins DJ, Dunlop MG . 1998 Am. J. Hum. Genet. 63: 749–759
Georgiades IB, Curtis LJ, Morris RM, Bird CC, Wyllie AH . 1999 Oncogene 18: 7933–7940
Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, Redston M, Gallinger S . 2000 N. Engl. J. Med. 342: 69–77
Hawn MT, Umar A, Carethers JM, Marra G, Kunkel TA, Boland CR, Koi M . 1995 Cancer Res. 55: 3721–3725
Ho JW, Yuen ST, Chung LP, Kwan KY, Chan TL, Leung SY, Chan AS, Tse C, Lam PW, Luk IS . 2000 Int. J. Cancer 89: 356–360
Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M . 1993 Nature 363: 558–561
Kallioniemi A, Kallioniemi OP, Sudar D, Rutovitz D, Gray JW, Waldman F, Pinkel D . 1992 Science 258: 818–821
Konishi M, Kikuchi-Yanoshita R, Tanaka K, Muraoka M, Onda A, Okumura Y, Kishi N, Iwama T, Mori T, Koike M, Ushio K, Chiba M, Nomizu S, Konishi F, Utsunomiya J, Miyaki M . 1996 Gastroenterology 111: 307–317
Lengauer C, Kinzler KW, Vogelstein B . 1997 Nature 386: 623–627
Lengauer C, Kinzler KW, Vogelstein B . 1998 Nature 396: 643–649
Leung SY, Yuen ST, Chan TL, Chan AS, Ho JW, Kwan K, Fan YW, Hung KN, Chung LP, Wyllie AH . 2000 Oncogene 19: 4079–4083
Liu B, Farrington SM, Petersen GM, Hamilton SR, Parsons R, Papadopoulos N, Fujiwara T, Jen J, Kinzler KW, Wyllie AH, Vogelstein B, Dunlop MG . 1995 Nat. Med. 1: 348–352
Liu B, Parsons R, Papadopoulos N, Nicolaides NC, Lynch HT, Watson P, Jass JR, Dunlop M, Wyllie A, Peltomaki P, de la Chapelle A, Hamilton SR, Vogelstein B, Kinzler KW . 1996 Nat. Med. 2: 169–174
Lothe RA, Peltomaki P, Meling GI, Aaltonen LA, Nystrom-Lahti M, Pylkkanen L, Heimdal K, Andersen TI, Moller P, Rognum TO, Fossa SD, Haldorsen T, Langmark F, Brogger A, de la Chapelle A, Borresen AL . 1993 Cancer Res. 53: 5849–5852
Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, Hall PA . 1994 Cancer Res. 54: 614–617
Miyazaki M, Furuya T, Shiraki A, Sato T, Oga A, Sasaki K . 1999 Cancer Res. 59: 5283–5285
Muleris M, Salmon RJ, Dutrillaux B . 1988 Cancer Genet. Cytogenet. 32: 43–50
Muleris M, Salmon RJ, Dutrillaux B . 1990 Cancer Genet. Cytogenet. 46: 143–156
Olschwang S, Hamelin R, Laurent-Puig P, Thuille B, De Rycke Y, Li YJ, Muzeau F, Girodet J, Salmon RJ, Thomas G . 1997 Proc. Natl. Acad. Sci. USA 94: 12122–12127
Peltomaki P, Lothe RA, Aaltonen LA, Pylkkanen L, Nystrom-Lahti M, Seruca R, David L, Holm R, Ryberg D, Haugen A, Brogger A, Borresen AL, de la Chapelle A . 1993 Cancer Res. 53: 5853–5855
Remvikos Y, Vogt N, Muleris M, Salmon RJ, Malfoy B, Dutrillaux B . 1995 Genes Chromosomes Cancer 12: 272–276
Ried T, Knutzen R, Steinbeck R, Blegen H, Schrock E, Heselmeyer K, du Manoir S, Auer G . 1996 Genes Chromosomes Cancer 15: 234–245
Schlegel J, Stumm G, Scherthan H, Bocker T, Zirngibl H, Ruschoff J, Hofstadter F . 1995 Cancer Res. 55: 6002–6005
Thibodeau SN, Bren G, Schaid D . 1993 Science 260: 816–819
Toft NJ, Winton DJ, Kelly J, Howard LA, Dekker M, te Riele H, Arends MJ, Wyllie AH, Margison GP, Clarke AR . 1999 Proc. Natl. Acad. Sci. USA 96: 3911–3915
Yuen ST, Chung LP, Leung SY, Luk IS, Chan SY, Ho JC, Ho JW, Wyllie AH . 1997 Br. J. Cancer 76: 1610–1616
Acknowledgements
This work was supported by the Research Grants Council of the Hong Kong Special Administrative Region (HKU 7330/00M), the Committee on Research and Conference Grant from the University of Hong Kong (10202796), a donation from the Hong Kong Society of Gastroenterology, grants from the Cancer Research Campaign (SP2326/0101) and Scottish Health Department (K/MRS/50/C2417, C2723), and the Scottish Hospitals Endowments Research Trust (#1263).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chan, T., Curtis, L., Leung, S. et al. Early-onset colorectal cancer with stable microsatellite DNA and near-diploid chromosomes. Oncogene 20, 4871–4876 (2001). https://doi.org/10.1038/sj.onc.1204653
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204653
Keywords
This article is cited by
-
Reply to ‘Comment on ‘Distinct clinical outcomes of two CIMP-positive colorectal cancer subtypes based on a revised CIMP classification system”
British Journal of Cancer (2018)
-
Colorectal Cancer in the Young
Current Gastroenterology Reports (2018)
-
The Rising Incidence of Younger Patients With Colorectal Cancer: Questions About Screening, Biology, and Treatment
Current Treatment Options in Oncology (2017)
-
Copy number alterations and allelic ratio in relation to recurrence of rectal cancer
BMC Genomics (2015)
-
Molecular Origins of Colon and Rectal Cancer: Not a Wnt–Wnt Situation
Current Colorectal Cancer Reports (2013)