Abstract
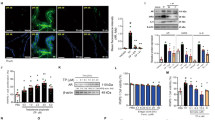
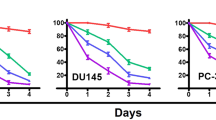
Prostate cancer is one of the most common cancers in men and it is the second leading cause of cancer related death in men in the United States. Recent dietary and epidemiological studies have suggested the benefit of dietary intake of fruits and vegetables in lowering the incidence of prostate cancer. A diet rich in fruits and vegetables provides phytochemicals, particularly indole-3-carbinol (I3C), which may be responsible for the prevention of many types of cancer, including hormone-related cancers such as prostate. Studies to elucidate the role and the molecular mechanism(s) of action of I3C in prostate cancer, however, have not been conducted. In the current study, we investigated whether I3C had any effect against prostate cancer cells and, if so, attempts were made to identify the potential molecular mechanism(s) by which I3C elicits its biological effects on prostate cancer cells. Here we report for the first time that I3C inhibits the growth of PC-3 prostate cancer cells. Induction of G1 cell cycle arrest was also observed in PC-3 cells treated with I3C, which may be due to the observed effects of I3C in the up-regulation of p21WAF1 and p27Kip1 CDK inhibitors, followed by their association with cyclin D1 and E and down-regulation of CDK6 protein kinase levels and activity. The induction of p21WAF1 appears to be transcriptionally upregulated and independent of the p53 responsive element. In addition, I3C inhibited the hyperpohosphorylation of the Retinoblastoma (Rb) protein in PC-3 cells. Induction of apoptosis was also observed in this cell line when treated with I3C, as measured by DNA laddering and poly (ADP-ribose) polymersae (PARP) cleavage. We also found an up-regulation of Bax, and down-regulation of Bcl-2 in I3C-treated cells. These effects may also be mediated by the down-regulation of NF-κB observed in I3C treated PC-3 cells. From these results, we conclude that I3C inhibits the growth of PC-3 prostate cancer cells by inducing G1 cell cycle arrest leading to apoptosis, and regulates the expression of apoptosis-related genes. These findings suggest that I3C may be an effective chemopreventive or therapeutic agent against prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
ACS. 2000 Cancer facts and figures American Cancer Society Inc.: Atlanta, GA
Bailey G, Selivonchick D, Hendricks J . 1987 Environ. Health Perspect. 71: 147–153
Beier RC . 1990 Rev. Environ. Contam. Toxicol. 113: 47–137
Biggs JR, Kraft AS . 1999 J. Biol. Chem. 274: 36987–36994
Biggs JR, Kudlow JE, Kraft AS . 1996 J. Biol. Chem. 271: 901–906
Bradfield CA, Bjeldanes LF . 1991 Adv. Exp. Med. Biol. 289: 153–163
Broadbent TA, Broadbent HS . 1998 Curr. Med. Chem. 5: 469–491
Cohen JH, Kristal AR, Stanford JL . 2000 J. Natl. Cancer Inst. 92: 61–68
Costello JF, Plass C, Arap W, Chapman VM, Held WA, Berger MS, Su Huang HJ, Cavenee WK . 1997 Cancer Res. 57: 1250–1254
Cover CM, Hsieh SJ, Tran SH, Hallden G, Kim GS, Bjeldanes LF, Firestone GL . 1998 J. Biol. Chem. 273: 3838–3847
Cram EJ, Garcia HH, Cover CM, Bjeldanes LF, Firestone GL . 2000 American Association of Cancer Research 91 San Francisco, CA p. 10
Darmon AJ, Nicholson DW, Bleackley RC . 1995 Nature 377: 446–448
Dashwood RH . 1998 Chem. Biol. Interact. 110: 1–5
Dashwood RH, Arbogast DN, Fong AT, Pereira C, Hendricks JD, Bailey GS . 1989 Carcinogenesis 10: 175–181
Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF . 1995a Proc. Natl. Acad. Sci. USA 92: 5545–5549
Datto MB, Yu Y, Wang XF . 1995b J. Biol. Chem. 270: 28623–28628
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B . 1993 Cell 75: 817–825
Gartel AL, Serfas MS, Gartel M, Goufman E, Wu GS, el-Deiry WS, Tyner AL . 1996 Exp. Cell. Res. 227: 171–181
Ge X, Yannai S, Rennert G, Gruener N, Fares FA . 1996 Biochem. Biophys. Res. Commun. 228: 153–158
Grubbs CJ, Steele VE, Casebolt T, Juliana MM, Eto I, Whitaker LM, Dragnev KH, Kelloff GJ, Lubet RL . 1995 Anticancer Res. 15: 709–716
Guo D, Schut HA, Davis CD, Snyderwine EG, Bailey GS, Dashwood RH . 1995 Carcinogenesis 16: 2931–2937
Huang P, Ballal K, Plunkett W . 1997 Cancer Res. 57: 3407–3414
Huber WW, McDaniel LP, Kaderlik KR, Teitel CH, Lang NP, Kadlubar FF . 1997 Mutat. Res. 376: 115–122
Katdare M, Osborne MP, Telang NT . 1998 Oncol. Rep. 5: 311–315
Kelloff GJ, Boone CW, Crowell JA, Steele VE, Lubet RA, Doody LA, Malone WF, Hawk ET, Sigman CC . 1996 J. Cell. Biochem. Suppl. 26: 1–28
Kim DJ, Han BS, Ahn B, Hasegawa R, Shirai T, Ito N, Tsuda H . 1997 Carcinogenesis 18: 377–381
Kojima T, Tanaka T, Mori H . 1994 Cancer Res. 54: 1446–1449
Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, John EM, Howe GR, Dreon DM, West DW, Paffenbarger Jr RS . 2000 Cancer Epidemiol. Biomarkers Prev. 9: 795–804
Krongrad A, Lai S, Vidal EM . 1998 Semin. Urol. Oncol. 16: 30–34
Liu M, Iavarone A, Freedman LP . 1996a J. Biol. Chem. 271: 31723–31728
Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP . 1996b Genes Dev. 10: 142–153
Lukas J, Bartkova J, Rohde M, Strauss M, Bartek J . 1995a Mol. Cell. Biol. 15: 2600–2611
Lukas J, Parry D, Aagaard L, Mann DJ, Bartkova J, Strauss M, Peters G, Bartek J . 1995b Nature 375: 503–506
Michnovicz JJ, Adlercreutz H, Bradlow HL . 1997 J. Natl. Cancer Inst. 89: 718–723
Missero C, Calautti E, Eckner R, Chin J, Tsai LH, Livingston DM, Dotto GP . 1995 Proc. Natl. Acad. Sci. USA 92: 5451–5455
Niwa T, Swaneck G, Bradlow HL . 1994 Steroids 59: 523–527
Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM . 1994 Nature 372: 570–573
Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M . 1995 Mol. Cell. Biol. 15: 2612–2624
Parker SB, Eichele G, Zhang P, Rawls A, Sands AT, Bradley A, Olson EN, Harper JW, Elledge SJ . 1995 Science 267: 1024–1027
Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A . 1994a Genes Dev. 8: 9–22
Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J . 1994b Cell 78: 59–66
Safe SH . 1995 Environ. Health Perspect. 103: 346–351
Sakr WA, Grignon DJ, Haas GP, Schomer KL, Heilbrun LK, Cassin BJ, Powell J, Montie JA, Pantes JE, Crissman JD . 1995 Pathol. Res. Pract. 191: 838–841
Salomons GS, Brady HJ, Verwijs-Janssen M, Van Den Berg JD, Hart AA, Van Den Berg H, Behrendt H, Hahlen K, Smets LA . 1997 Int. J. Cancer 71: 959–965
Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, Korsmeyer SJ . 1995 Proc. Natl. Acad. Sci. USA 92: 7834–7838
Sherr CJ . 1994 Cell 79: 551–555
Sherr CJ, Roberts JM . 1995 Genes Dev. 9: 1149–1163
Slingerland JM, Hengst L, Pan CH, Alexander D, Stampfer MR, Reed SI . 1994 Mol. Cell. Biol. 14: 3683–3694
Steinmetz KA, Potter JD . 1996 J. Am. Diet. Assoc. 96: 1027–1039
Teixeira C, Pratt MA . 1997 Mol. Endocrinol. 11: 1191–1202
Telang NT, Katdare M, Bradlow HL, Osborne MP, Fishman J . 1997 Proc. Soc. Exp. Biol. Med. 216: 246–252
Timmermann S, Hinds PW, Munger K . 1997 Cell Growth Differ. 8: 361–370
Tiwari RK, Guo L, Bradlow HL, Telang NT, Osborne MP . 1994 J. Natl. Cancer Inst. 86: 126–131
Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM . 1996 Science 274: 787–789
Wang CY, Mayo MW, Baldwin Jr AS . 1996 Science 274: 784–787
Wong GY, Bradlow L, Sepkovic D, Mehl S, Mailman J, Osborne MP . 1997 J. Cell. Biochem. Suppl. 29: 111–116
Xu M, Bailey AC, Hernaez JF, Taoka CR, Schut HA, Dashwood RH . 1996 Carcinogenesis 17: 1429–1434
Acknowledgements
This work was partly funded by the George Puschelberg Foundation and we sincerely thank the Foundation for its support. We also sincerely thank Ms Patricia Arlauskas for her editorial assistance.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chinni, S., Li, Y., Upadhyay, S. et al. Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene 20, 2927–2936 (2001). https://doi.org/10.1038/sj.onc.1204365
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204365
Keywords
This article is cited by
-
The role of diet in renal cell carcinoma incidence: an umbrella review of meta-analyses of observational studies
BMC Medicine (2022)
-
In-vivo antiproliferative activity of Morus latifolia leaf and bark extracts against Ehrlich’s ascites carcinoma
Toxicological Research (2020)
-
Effects of indole-3-carbinol on steroid hormone profile and tumor progression in a mice model of canine inflammatory mammarycancer
BMC Cancer (2018)
-
PLGA encapsulation and radioiodination of indole-3-carbinol: investigation of anticancerogenic effects against MCF7, Caco2 and PC3 cells by in vitro assays
Journal of Radioanalytical and Nuclear Chemistry (2017)
-
NFκBP65 transcription factor modulates resistance to doxorubicin through ABC transporters in breast cancer
Breast Cancer (2017)