Abstract
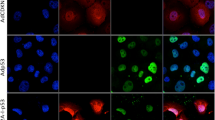
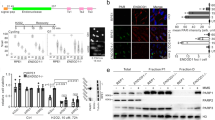
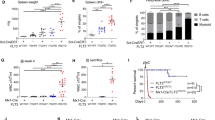
The high prevalence and great diversity of p53 tumor suppressor gene mutations in human tumors call for development of therapeutic molecules that rescue function of aberrant p53 protein. P53 mutations also offer new approaches to the study of the origins of mutations in human cancer. An experimental mouse model with a genetically modified but normal functioning p53 gene harboring the human rather than the murine core domain, would be of considerable benefit to research on both cancer therapeutics and etiology; however, it is uncertain whether such mice would permit biological functions of p53 to be retained. Using a Cre/lox P gene-targeting approach, we have constructed a human p53 knock-in (hupki) mouse strain in which exons 4–9 of the endogenous mouse p53 allele were replaced with the homologous, normal human p53 gene sequence. The chimeric p53 allele (p53KI) is properly spliced, transcribed in various tissues at levels equivalent to wild-type mice, and yields cDNA with the anticipated sequence, that is, with a core domain matching that of humans. The hupki p53 protein binds to p53 consensus sequences in gel mobility shift assays and accumulates in the nucleus of hupki fibroblasts in response to UV irradiation, as is characteristic of wild-type p53. Induction of various p53-regulated genes in spleen of γ-irradiated homozygous hupki mice (p53KI/KI), and the kinetics of p53-dependent apoptosis in thymocytes are similar to results with wild-type (p53+/+) mice, further indicating normal p53 pathway function in the hupki strain. The mice are phenotypically normal and do not develop spontaneous tumors at an early age, in contrast to knock-out (p53−/−) strains with a defective p53 gene. The chimeric (p53KI) allele thus appears to provide a biological equivalent to the endogenous murine (p53+) gene. This strain is a unique tool for examining in vivo spontaneous and induced mutations in human p53 gene sequences for comparison with published human tumor p53 mutation spectra. In addition, the hupki strain paves the way for mouse models in pre-clinical testing of pharmaceuticals designed to modulate DNA-binding activity of human p53.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Abbreviations
- hupki :
-
human p53 knock-in mice
- KI:
-
knock-in allele
References
Aguilar F, Hussain SP and Cerutti P. . 1993 Proc. Natl. Acad. Sci. USA 90: 8586–8590.
Albrechtsen N, Dornreiter I, Grosse F, Kim E, Wiesmuller L and Deppert W. . 1999 Oncogene 18: 7706–7717.
Baker SJ, Preisinger AC, Jessup JM, Paraskeva C, Markowitz S, Willson JKV, Hamilton S and Vogelstein B. . 1991 Cancer Res. 50: 7717–7722.
Beckman KB and Ames BN. . 1998 Physiol. Rev. 78: 547–581.
Brachmann RK, Yu K, Eby Y, Pavletich NP and Boeke JD. . 1998 EMBO J. 17: 1847–1859.
Cho Y, Gorina S, Jeffrey PD and Pavletich NP. . 1994 Science 265: 346–355.
Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML and Wyllie AH. . 1993 Nature 362: 849–852.
Denissenko MF, Pao A, Tang M and Pfeifer GP. . 1996 Science 274: 430–432.
Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Butel JS and Bradley A. . 1992 Nature 356: 215–221.
Dudenhoffer C, Kurth M, Janus F, Deppert W and Wiesmuller L. . 1999 Oncogene 18: 5773–5784.
Dumaz N, van Kranen HJ, de Vries A, Berg RJW, Wester PW, van Kreijl CF, Sarasin A, Daya-Grosjean L and deGruijl FR. . 1997 Carcinogenesis 18: 897–904.
Flaman JM, Frebourg T, Moreau F, Charbonnier C, Martin C, Ishioka C, Friend SH & Iggo R. . 1994 Nucleic Acids Res. 22: 3259–3260.
Foster BA, Coffey HA, Morin MJ, Rastinejad F. . 1999 Science 286: 2507–2510.
Hainaut P and Hollstein M. . 2000 Adv. Cancer Res. 77: 81–137.
Harris CC and Hollstein M. . 1993 New Engl. J. Med. 329: 1318–1327.
Hollstein M, Hergenhahn M, Yang Q, Bartsch H, Wang Z-Q and Hainaut P. . 1999 Mutat. Res. 431: 199–209.
Hollstein M, Moeckel G, Hergenhahn M, Spiegelhalder B, Keil M, Werle-Schneider G, Bartsch H and Brickman J. . 1998 Mutation Res. 405: 145–154.
Hupp TR, Sparks Z and Lane DP. . 1995 Cell 83: 237–245.
Hurstings SD, Perkins SN, Haines DC, Ward JM and Phang JM. . 1995 Cancer Res. 55: 3949–3953.
Jacks T, Remington BO, Williams EM, Schmitt S, Halachmi S, Bronson RT and Weinberg RA. . 1994 Curr. Biol. 4: 1–7.
Kleihues P, Schaeuble B, zur Hausen A, Esteve J and Ohgaki H. . 1997 Am. J. Pathology 150: 1–13.
Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov, MV and Gudkov AW. . 1999 Science 285: 1733–1737.
Lane DP. . 1999 Br. J. Cancer 80: 1–5.
Lane DP, Stephen CW, Midgley CA, Sparks A, Hupp TR, Daniels DA, Greaves R, Reid A, Vojtesek B and Picksley SM. . 1996 Oncogene 12: 2461–2466.
Lehman TA, Bennett WP, Metcalf RA, Welsh JA, Ecker J, Modali R, Ullrich S, Romano JW, Appella E, Testa JR, Gerwin BI and Harris CC. . 1991 Cancer Res. 51: 4090–4096.
Liu G, McDonnell TJ, Montes de Oca Luna R, Kapoor M, Mims B, El-Naggar AK and Lozano G. . 2000 Proc. Natl. Acad. Sci. USA 97: 4174–4179.
Lowe SW, Schmitt EM, Smith SW, Osborne BA and Jacks T. . 1993 Nature 362: 847–852.
Marin MC, Jost CA, Brooks LA, Irwin MS, O'Nions J, Tidy JA, James N, McGregor JM, Harwood CA, Yulug IG, Vousden KH, Allday MJ, Gusterson B, Ikawa S, Hinds PW, Crook T and Kaelin WG. . 2000 Nature Genetics 25: 47–53.
Prives C and Hall PA. . 1999 J. Pathol. 187: 112–126.
Rodriguez MS, Desterro JMP, Lain S, Midgley CA, Lane DP and Hay RT. . 1999 EMBO J. 18: 6455–6461.
Selivanova G, Iotosova WV, Okan I, Fritsche M, Strom M, Groner B, Grafstrom RC and Wiman KG. . (1997). Nature Medicine 3: 632–638.
Shamser M and Montano X. . 1996 Gene 176: 259–262.
Ushijima T, Makino H, Okonogi H, Hosoya Y, Sugimura T and Nagao M. . 1995 Mol. Carcinogenesis 12: 23–30.
Vogelstein B and Kinzler KW. . 1992 Cell 70: 523–526.
Wang ZQ, Auer B, Stingl L, Berghammer H, Haidacher D, Schweiger M and Wagner EF. . 1995 Genes and Dev. 9: 509–520.
Yu J, Zhang L, Hwang PM, Rago C, Kinzler KW and Vogelstein B. . 1999 Proc. Natl. Acad. Sci. USA 96: 14517–14522.
Zhang Z, Liu Q, Lantry LE, Wang Y, Kelloff GJ, Anderson MW, Wiseman RW, Lubet RA and You M. . 2000 Cancer Res. 60: 901–907.
Zhao R, Gish K, Murphy M, Yin Y, Notterman D, Hoffman WH, Tom E, Mack DH and Levine AJ. . 2000 Genes and Development 14: 981–993.
Acknowledgements
This study was supported by grant number R01CA79493 from the National Cancer Institute (NCI, USA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute (NCI). We are grateful to Dr R Koomagi for immunocytochemistry studies, and thank U Schmitt, K-R Muehlbauer, Joslyn Michelon and Dominique Galendo for technical assistance.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Luo, JL., Yang, Q., Tong, WM. et al. Knock-in mice with a chimeric human/murine p53 gene develop normally and show wild-type p53 responses to DNA damaging agents: a new biomedical research tool. Oncogene 20, 320–328 (2001). https://doi.org/10.1038/sj.onc.1204080
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204080
Keywords
This article is cited by
-
African-centric TP53 variant increases iron accumulation and bacterial pathogenesis but improves response to malaria toxin
Nature Communications (2020)
-
Humanising the mouse genome piece by piece
Nature Communications (2019)
-
Mutant p53 drives clonal hematopoiesis through modulating epigenetic pathway
Nature Communications (2019)
-
Modelling mutational landscapes of human cancers in vitro
Scientific Reports (2014)
-
Two hot spot mutant p53 mouse models display differential gain of function in tumorigenesis
Cell Death & Differentiation (2013)